Hydrogen Sulfide:
H2S
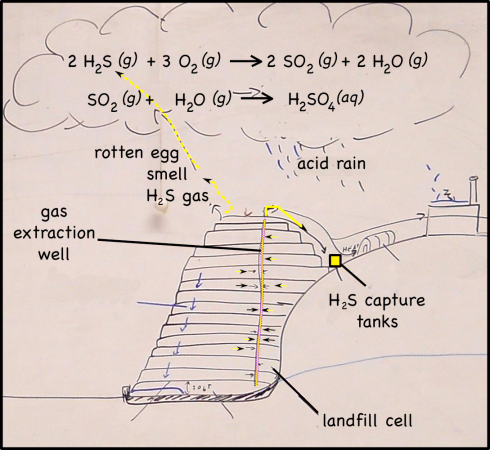
Although it makes up less than 1% of the gases produced by landfills, hydrogen sulfide (H2S) is the major reason landfills smell as bad as they do. H2S is produced by decomposition in the landfill, and if it’s not captured it not only produces a terrible, rotten-egg smell, but also produces acid rain, and, in high enough concentrations, it can be harmful to your health (OSHA, 2005; Ohio Dept. Health, 2010).
Decomposition

Some hydrogen sulfide is produced when organic matter decays, but for big landfills like the one we visited, construction materials, especially gypsum wallboard (drywall), are probably the biggest source.
Gypsum is a calcium sulphate mineral, that’s made into sheets of drywall that are used cover the walls in most houses because it’s easy to work with and retards fire. The U.S. used 17 million tons of gypsum for drywall in 2010 according to the USGS’s Mineral Commodity Summary (USGS, 2011 (pdf)).
Gypsum:
CaSO4•2(H2O)
As you can see from the chemical formula, each gypsum molecule has two water molecules attached. In a fire, the heat required to evaporate the water keeps the temperature of the walls down to only 100 degrees Celcius until the water has evaporated out of the gypsum board.
A number of landfills have banned drywall because it produces so much hydrogen sulfide, but the one we visited still takes it. It’s big enough that they capture the landfill gasses, including the hydrogen sulfide, and then separate it from the other, more useful gasses, like methane, which can be burned to produce heat energy. H2S can also be burned, but they you end up contributing to acid rain.
H2S and Acid Rain
When hydrogen sulfide reacts with oxygen in the atmosphere it produces sulfur dioxide.
2 H2S (g) + 3 O2 (g) —-> 2 SO2 (g) + 2 H2O (g)
Sulfur dioxide, in turn, reacts with water droplets in clouds to create sulfuric acid.
SO2 (g) + H2O (g) —-> H2SO4 (aq)

When those droplets eventually coalesce into raindrops, they will be what we call acid rain.
Acid rain damages ecosystems and dissolves statues. It used to be a major problem in the midwestern and eastern United States, but in 1995 the EPA started a cap and trade program for sulfur dioxide emissions (remember sulfur dioxide is produced by burning hydrogen sulfide) that has made a huge difference.

Capturing H2S
Probably because of the EPA’s restrictions, the landfill company pipes all the gases it collects through scrubbers to extract the hydrogen sulfide. There are a few ways to capture H2S, they all involve running the gas through a tank of some sort of scavenging system that holds a chemical that will react with hydrogen sulfide and not the other landfill gases. At the landfill we visited the remaining landfill gas, which consisted of mostly methane, was used for its energy.