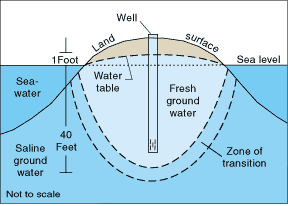
Rain falls.
Some runs off,
Some seeps into the ground.

It trickles through soil.
Leaching acids, organic,
Out of the leaf litter,
But even without these,
It’s already, every so slightly, corrosive,
From just the carbon dioxide in the air.
Gravity driven,
The seeping water seeks the bedrock,
Where it might find,
In the Ozark Mountains,
Limestone.

Limestone:
Microscopic shells, of plankton,
Raining down, over millenia,
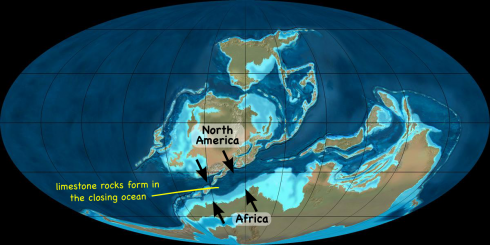
Compacting into rocks,
In a closing ocean,
As North America and Africa collide,
From the Devonian to the Carboniferous.
Orogenic uplift,
Ocean-floor rocks,
Become mountains,
Appalachians, Ouachitas,
The Ozark Plateau.
Limestone dissolves,
In acid water.
Shaping holes; caves in bedrock,
Where we go,
Exploring.