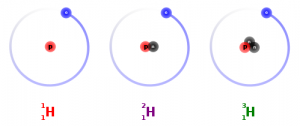
I have been told by reliable sources that the difference between atomic mass and atomic weight is that the atomic mass is the mass of a single atom (number of protons plus the number of neutrons), while the atomic weight is the averaged masses of all the different isotopes you would find in a natural sample.
This obviously requires a discussion of isotopes, which may be a topic best left for high school. However there are a number of interesting hooks that could capture the imagination.
One of them is the use of isotopes to trace sources of your diet. Isotopes can tell how much meat a person eats (nitrogen-15) or how much of the carbon in their body comes, ultimately, from corn.