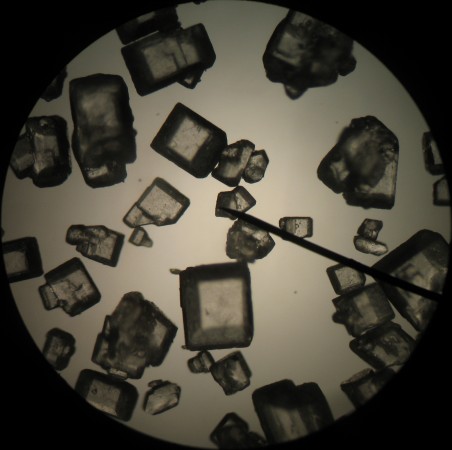
Salt and sugar crystals have wonderfully distinctive crystal forms. They might well be good subjects for introducing minerals, crystals and some of the more complex geometric solids.
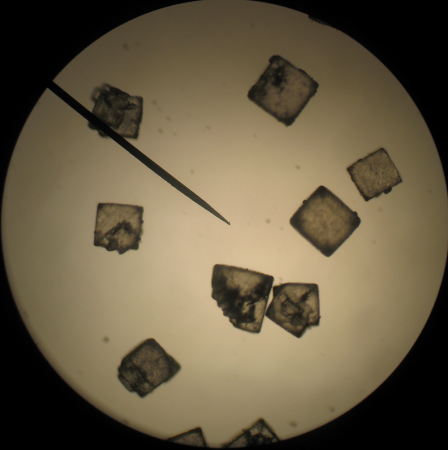
The salt crystals are clearly cubic, even though some of the grains seem to be made up of overlapping cubes.

Salt is an ionic compound, made of sodium and chloride atoms (NaCl). When a number of these molecules get together to form a crystal, they tend to arrange themselves in a cubic pattern. As a result, the salt crystals are also cubic. In fact, if you break a salt crystal, it will tend to break along the planes that are at the surfaces of the planes of the atomic lattice to create a nice, shiny crystal faces. Gem cutters use this fact to great effect when they shape diamonds and other precious stones.
Of course different crystals have different atomic arrangements. The difference is clear when you compare salt to sugar.
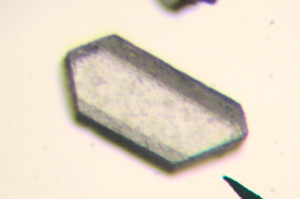
Sugar crystals look a bit like hexagonal pillars that have fallen over. According to the Beet-sugar handbook (Asadi, 2007), sugar crystals actually have a monoclinic form, which could end up as asymmetric hexagonal pillars. Salt crystals, on the other hand, have the habit of forming cubes.
I would like to know how you could tell the difference between salt and sugar crystals? How could you tell the difference without tasting them?
Sydney Capodano: I would like to know how you could tell the difference between salt and sugar crystals? How could you tell the difference without tasting them?
Other than with a microscope, I’m not sure. You might be able to distinguish the shapes with a hand lens. The salt crystals make almost perfect cubes and when they break, they will tend to fracture at 90 degree angles. Sugar crystals on the other hand will have shapes and angles that are more “rounded” (greater than 90 degrees). They should look almost hexagonal.
If you can dissolve them, you could also measure their electrical conductivity. A simple test would be to connect two wires to a small battery and stick them in the solution (not touching each other). The salt solution should create bubbles because it dissociates the NaCl. There should not be any reaction with the sugar solution since sugar is not an ionic compound like salt is.
There must be other tests though if you have to distinguish them as solids. I would hypothesize that if you poured two piles, one of salt and one of sugar, the angle of the slope that naturally forms (the angle of repose) would be different because of the different crystal shapes. They probably also have different hardnesses so maybe one is easier to crush than the other. Figuring these out would make for some interesting experiments.