
Elements can be identified from the color of light they give off when they’re ionized: their emission spectra. Ms. Wilson’s chemistry class today set fire to some metal salts to watch them burn.
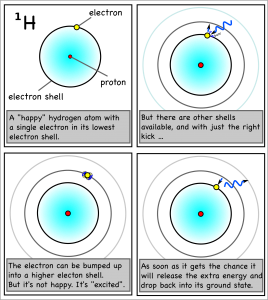
She placed the salt crystals into petri dishes, submerged them in a shallow layer of alcohol, and ignited the alcohol. As traces of the salts were incorporated into the flames, the metal atoms became “excited” as they absorbed some of the energy from the flame by bumping up their electrons into higher electron shells. Since atoms don’t “like” to be excited, their excited electrons quickly dropped back to their stable, ground state, but, in doing so, released the excess energy as light of the characteristic wavelength.
Table 1: Emission colors of different metals.
| Metal | Flame |
|---|---|
| Copper |  |
| Strontium | 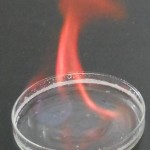 |
Sodium |  |
Lithium |  |