Having demonstrated how to draw a few simple atoms, I had students fill out a periodic table template with drawings of the first twenty atoms. Actually, I only had them draw the electrons in their shells because it reduced the messiness of trying to fit in forty nuclear particles into a small tile, and the point I wanted to get at was the pattern of shells and valence electrons in the periodic table.
The end result looked something like this:
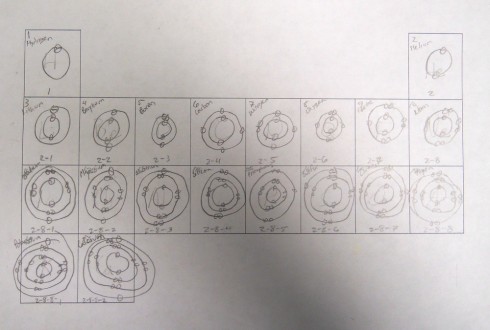
All the drawing only took about 15 minutes, and once they’d figured out the first half dozen or so it started to get a little boring. But that freed up the cognitive resources so they could notice the two key patterns.
- First, each row in the periodic table has an additional electron shell.
- Second, as you go across a row you add one electron to the shell until it is filled.
It’s a first glimpse at the periodicity in the periodic table. And it sets us up nicely to be able to talk about chemical bonding.