Now that we’ve learned how to draw individual atoms (and have an online reference for the first 20 elements), let’s consider ionic bonding.
The key thing to remember is that atoms all “want” to have their outer electron shells filled. So while a sodium (symbol: Na) atom is happy* enough that it has the same number of protons and electrons (11 each) it could be happier if it got rid of the extra electron in it’s outer shell.
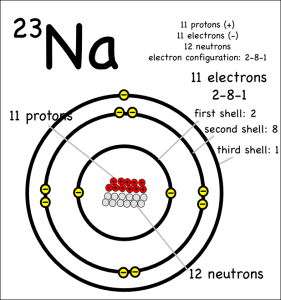
It can get rid of the electron by donating it to another atom that would be happier with an extra eletron. Something like chlorine (symbol: Cl) that only has 7 electrons in its outer shell, but wants to have 8.
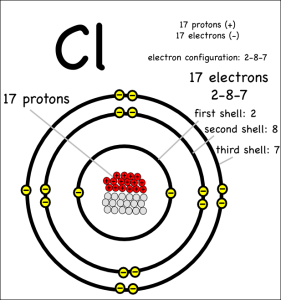
When one atom donates electrons to other atoms this creates a bond called an ionic bond. The molecule created is called an ionic molecule. In this case, sodium and chloride react to produce sodium chloride (chemical formula: NaCl).
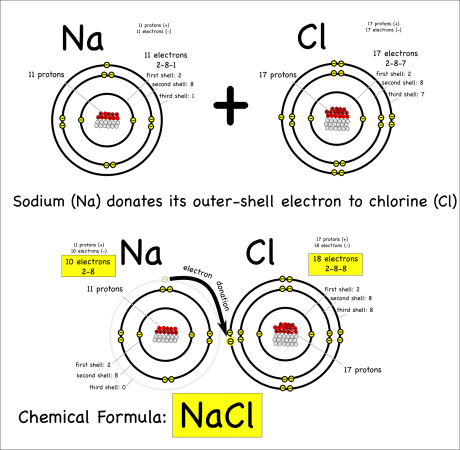
The chemical reaction can be written as:
Na + Cl –> NaCl
MgCl2
Now consider what happens when magnesium (symbol: Mg) reacts with chlorine.
Magnesium has two electrons in its outer shell that it wants to get rid of.
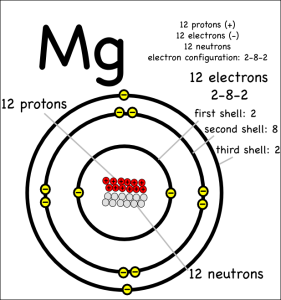
A single chlorine atom can’t take both, since chlorine only needs one electron to fill its outer electron shell. However, magnesium can give one electron to two different chlorine atoms to create a molecule with three atoms total.
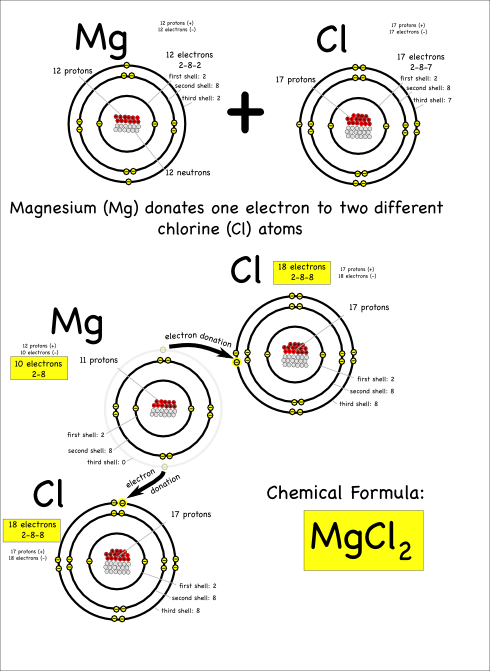
The resulting compound is called magnesium chloride, and is written as MgCl2. The subscripted 2 indicates that there are two chlorine atoms in each magnesium chloride molecule.
The chemical reaction can be written as:
Mg + 2 Cl –> MgCl2
Notice that each magnesium atom reacts with two chlorine atoms (Mg + 2 Cl) to produce a compound with one magnesium and two chlorines bonded together (chemical formula: MgCl2).
Practice
Now, for homework, you can try figuring out what is the chemical formula for the following ionic compounds:
- Potassium and Florine
- Beryllium and Chlorine
- Sodium and Oxygen
- Magnesium and Oxygen
Be sure to:
- Draw the atoms that you will be reacting,
- Show how the electrons are donated,
- Write the chemical formula of the resulting compound,
- Write the chemical reaction.
Good luck. Next we’ll try covalent bonding.
* Think of happiness as energy. Like people, atoms are happier to be in low energy states.
Notes
When we looked at the patterns in the periodic table, one of the things I had my student graph was the electronegativity. Electronegativity is the ability of atoms to attract electrons to themselves. You’ll note that chlorine’s electronegativity is high, while sodium’s is low.
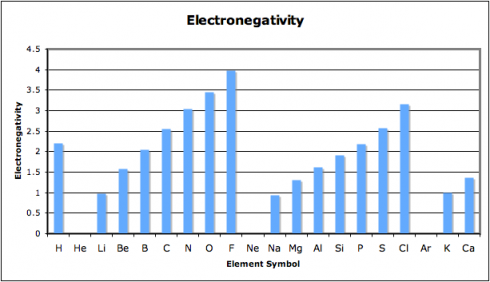
So chlorine will attract electrons to itself strongly, while sodium will not. This is why (more or less) sodium will end up donating its electron and why chlorine is happy to accept it.
When atoms with a large difference in electronegative bond together, the bonds tend to be ionic.