Covalent bonding happens when atoms share electrons, unlike with ionic bonding where one atom gives electrons to another.
Why do some combinations of atoms make ionic bonds and others covalent bonds? The answer has to do with electronegativity, which is the ability of atoms to attract electrons to themselves. Atoms that have similar abilities to attract electrons to themselves will likely form covalent bonds.
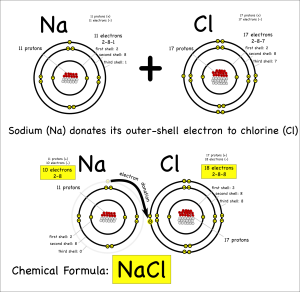
For either type of bond, the atoms have the same objective. All atoms “want” filled outer electron shells. When sodium reacts with chlorine for example, sodium has one electron in its outer shell and chlorine is one short of a filled outer shell so it’s “easiest” for sodium to just donate its electron to chlorine to make them both happy.
However, when two similar atoms bond it’s often easier to share electrons.
Consider two hydrogens bonding covalently to form hydrogen gas (note: help on drawing atoms).
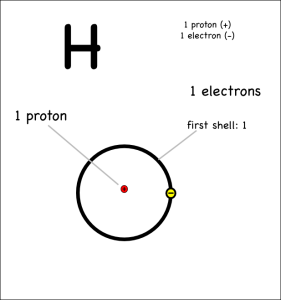
Each hydrogen has only one electron, and they both pull equally at the electrons so neither can give their electron away or take the other’s electron. Instead they share.
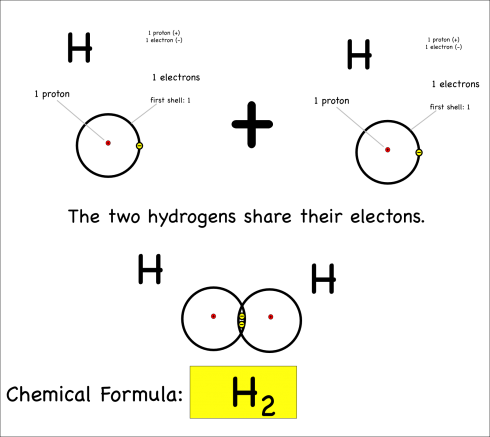
By sharing, they now each have two electrons in their outer shell, which is now full (since it’s the first shell), and both atoms are happy. This is covalent bonding.
The chemical reaction could be written as:
H + H –> H2
H2O
Now consider what happens when hydrogen atoms bond with oxygens. Oxygen atoms have 6 electrons in their outer shells, but they would like to have 8.
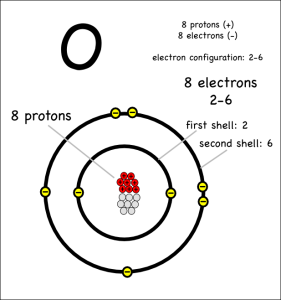
Oxygen atoms aren’t strong enough to take away the hydrogen electrons, so they share with covalent bonds. Each oxygen has to react with two hydrogens to get the two extra electrons it needs to end up with 8 electrons in its outer shell.
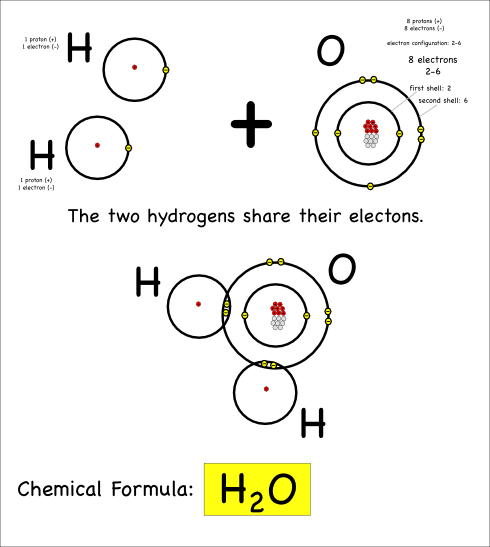
Thus we create water, which has the chemical formula H2O, and the chemical reaction can be written:
2 H + O –> H2O
Drawing covalent molecules
Covalent molecules can be large and complex, in fact, one strand of your DNA will have somewhere around a billion atoms.
To make these easier to draw, you can represent each element by its symbol and each bond by a line. Remember, each covalent bond represents a pair of electrons that are shared.
So our water molecule would be drawn like this:
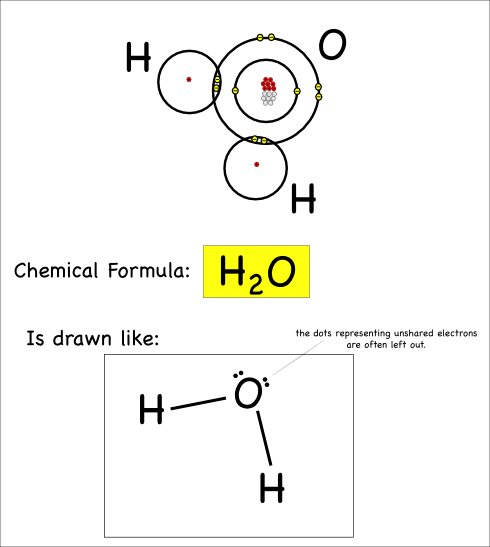
This is called a Lewis Dot structure. In addition to the lines showing the bonds, you’ll notice the dots that show the unbonded electrons: these dots are usually paired up.
Double Bonds
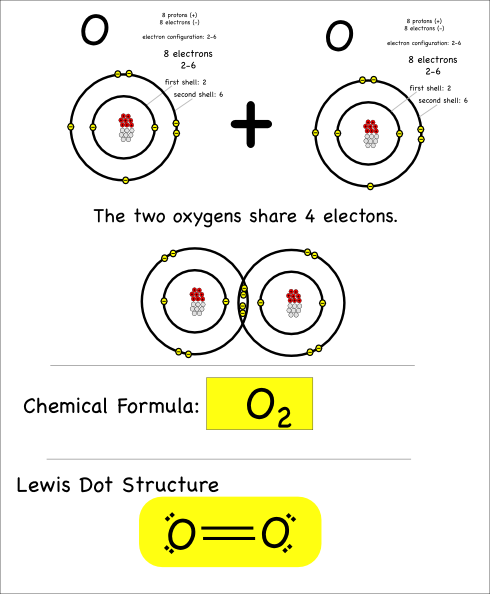
The last thing I’ll point out here is that atoms can share more than just one pair of electrons. When they share four electrons that means there are two bonds, which is referred to as a double bond.
Oxygen atoms bond with each other like this to make the oxygen gas we breathe.
Practice
Now you can try drawing these covalent molecules:
- A molecule with one nitrogen atom and some hydrogen atoms (can you figure out how many hydrogens)
- A molecule with the chemical formula: CH4
- A molecule with the chemical formula: C2H6
- A molecule with the chemical formula: C3H8
- A molecule with the chemical formula: C2H4 (hint there’s a double bond)
- A carbon dioxide molecule, which has the chemical formula: CO2
- An ozone molecule, which has the chemical formula: O3
- An alcohol molecule, which has the chemical formula: CH3OH