When we looked at the patterns in the periodic table, one of the things I had my student graph was the electronegativity. Electronegativity is the ability of atoms to attract electrons to themselves. You’ll note that chlorine’s electronegativity is high, while sodium’s is low.
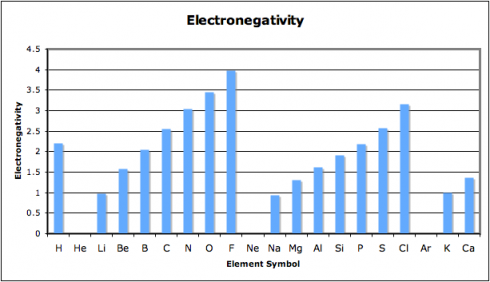
So chlorine will attract electrons to itself strongly, while sodium will not. This is why (more or less) sodium will end up donating its electron and why chlorine is happy to accept it.
When atoms with a large difference in electronegative bond together, the bonds tend to be ionic. On the other hand, when things with similar electronegativities bond together they tend to form covalent bonds.