The beautiful documentary “Cosmic Voyage“, is currently available for free on Hulu. It’s one of those things that sparks the imagination. UPDATE: At least it was on Hulu for a while. If I find it somewhere else I’ll post the link here.
Tag: atoms
Seeing temperature, kinetic energy and color
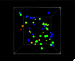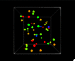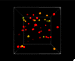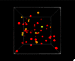
We read that temperature is the average kinetic energy of a substance but you can (especially if you’re a visual learner) nicely internalize this from simple videos or animations. UCAR has a little animation with their definition of temperature. I however, adapted an interactive, 3d animation that I think does a nice job, and also introduces a couple of other interesting concepts too.
I’ve also used this model, at different times, to show:
- The relationship between temperature and color emitted by objects. The main way we know the temperature of stars is because blue stars are hotter than red stars. Blue light has a shorter wavelength than red light, and things that are at higher temperatures emit shorter wavelengths.
- Absolute zero (0 Kelvin) – where (almost) all motion stops and the objects stop emitting light.
- Pressure in a gas – you really get a feel for the force exerted by the particles on the side of the box (although it might be even more interesting once I figure out how to add sound).
It is an interactive model, but it’s pretty simple because the only control is a slider that lets you set the temperature.
Finally, in the age of 3d movies, like Avatar, the models can be easily shown in 3d if you have the glasses (redcyan).
The model is easy to install and run on Windows, but you have to install the programming language VPython separately on a Mac (but that isn’t very hard). I have this, and a bunch of other models, at http://earthsciweb.org/GeoMod/.
Voyager

The Voyager spacecraft, launched in 1977, are still going and making new discoveries. They are after all the man-made objects that are furthest away from the center of the solar system, beyond the orbit of Pluto, and are now approaching interstellar space.
Where does the solar system end and interstellar space begin? Well, the Sun gives off light, but it also emits a plasma of charged particles (protons and electrons typically) that’s called the solar wind. These charged particles are launched from the Sun pretty fast, but as they get to the edge of the solar system they start to slow down, because the solar system is moving through a magnetic cloud, and, as we all know, charged particles are affected by magnetic fields.
The solar wind, assisted by the Sun’s magnetic field, pushes against the interstellar magnetic cloud, creating a bubble, called the heliosphere (helio=sun, sphere=sphere) that is pretty much the edge of the solar system.
Both Voyager spacecraft are approaching the heliosphere, and we’ve recently discovered that as the solar system moves through the interstellar magnetic cloud, the heliosphere is pushing against the cloud and the cloud is pushing back quite a bit. As a result, the heliosphere is shaped like the bow wave of water around a speeding boat.
It is difficult not to personify these two lonely spacecraft as the get further and further away from home, with no way to get back, but sending signals that tell of their discoveries and ensure their immortality.
How to fly through space

To get anywhere in the solar system takes a long time and is not easy. The Mars rovers took years to get there and were built to be very light because heavier spacecraft are harder to lift into orbit and take more energy to get them where they’re going (and to slow the down when they get there). Getting humans to other planets, or even another solar system, is an even greater challenge. New Scientist magazine has a nice little article on different types of deep-space spacecraft that might work.
Periodic table trivia
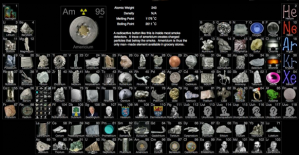
This online periodic table by Theodore Gray has pictures of all the elements. Some are in pure ingots or crystals, others in example applications, and some in ore form. What I find most interesting is there is a bit of trivia about each element when you hover over its picture. Also, the images of the noble gasses shows the colors they emit when excited with electricity (e.g. neon lights), which is kind of cool.
Nuclear Winter and MAD

Almost every time I discuss protons, neutrons and the nucleus of an atom, or at least so my students complain, I end up talking about nuclear fission and fusion and nuclear weapons. If the discussion goes on long enough I tend to bring up the cold war and how the fear of mutually assured destruction (MAD) reduced the chance of a hot war. I don’t often get into how the explosions from a nuclear exchange could put so much dust into the upper atmosphere that it blocks the sunlight and create a nuclear winter that would affect life all around the world. A nuclear winter that would have an effect similar to the winter created by the asteroid impact that lead to the extinction of the dinosaurs.
The danger of nuclear weapons have not, unfortunately, gone away. There is a facinating article in Scientific American on how even a “small” nuclear war could have global consequences. They have a great quote from Mikhail S. Gorbachev about how,
“Models made by Russian and American scientists showed that a nuclear war would result in a nuclear winter that would be extremely destructive to all life on earth; the knowledge of that was a great stimulus to us, to people of honor and morality, to act.”
The major finding of the research in the article is that even a small nuclear war, such as between India and Pakistan, could lead to a significant global nuclear winter.
I like to take every chance I get to tie natural and social world concepts together. It’s one of the things I enjoy most about teaching in an interdisciplinary Montessori classroom. There is a beautiful and scary story here about how the science of the infinitesimally small has had a fundamental effect on the major geopolitical conflict of the latter half of the 20th century, and continues to affect us today.
Atomic mass versus atomic weight
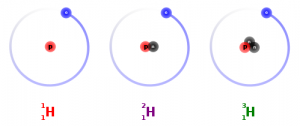
I have been told by reliable sources that the difference between atomic mass and atomic weight is that the atomic mass is the mass of a single atom (number of protons plus the number of neutrons), while the atomic weight is the averaged masses of all the different isotopes you would find in a natural sample.
This obviously requires a discussion of isotopes, which may be a topic best left for high school. However there are a number of interesting hooks that could capture the imagination.
One of them is the use of isotopes to trace sources of your diet. Isotopes can tell how much meat a person eats (nitrogen-15) or how much of the carbon in their body comes, ultimately, from corn.
Corn, chemistry and the food you eat

It’s absolutely amazing how much the different numbers of neutrons in atoms can tell us about the ourselves and the world. Over 99% of the carbon in the atmosphere is carbon-12, with 6 neutrons and 6 protons, but the rest is made of carbon-12 (6 protons and 7 neutrons) or carbon-14 (6 protons and 8 neutrons).
Carbon-14 is radioactive and is used to date things for archeology and climate change etc. However, when it comes to our diet carbon-13 is a bit more interesting. Some plants, particularly grasses like corn, do photosynthesis a little differently so that they tend to have more of the slightly heavier carbon-13 isotope than the others. As a result, if you take a blood sample, you can tell (roughly) how much of your diet ultimately came from grasses.
Why is this interesting? Because when you eat meat, there is a good chance that the animal you are eating was fed with corn. If you look at the pre-packaged items in the supermarket, you’ll find that high-fructose corn syrup is an important ingredient on many of them.
The documentary King Corn, and the book The Omnivore’s Dilemma find that if you trace the modern industrial food chain much of it starts in the corn fields of the mid-west. We eat, in one way or another, a lot of corn. In fact, blood samples have found over 50% of the carbon in our bodies comes from corn and similar grasses (like sugar cane).
This article describes a number of other interesting applications of isotopes in investigating diet. A more technical description of carbon-13 and diet can be found here.