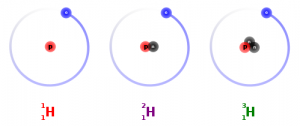
It’s absolutely amazing how much the different numbers of neutrons in atoms can tell us about the ourselves and the world. Over 99% of the carbon in the atmosphere is carbon-12, with 6 neutrons and 6 protons, but the rest is made of carbon-12 (6 protons and 7 neutrons) or carbon-14 (6 protons and 8 neutrons).
Carbon-14 is radioactive and is used to date things for archeology and climate change etc. However, when it comes to our diet carbon-13 is a bit more interesting. Some plants, particularly grasses like corn, do photosynthesis a little differently so that they tend to have more of the slightly heavier carbon-13 isotope than the others. As a result, if you take a blood sample, you can tell (roughly) how much of your diet ultimately came from grasses.
Why is this interesting? Because when you eat meat, there is a good chance that the animal you are eating was fed with corn. If you look at the pre-packaged items in the supermarket, you’ll find that high-fructose corn syrup is an important ingredient on many of them.
The documentary King Corn, and the book The Omnivore’s Dilemma find that if you trace the modern industrial food chain much of it starts in the corn fields of the mid-west. We eat, in one way or another, a lot of corn. In fact, blood samples have found over 50% of the carbon in our bodies comes from corn and similar grasses (like sugar cane).
This article describes a number of other interesting applications of isotopes in investigating diet. A more technical description of carbon-13 and diet can be found here.