A little stick of epoxy from the hardware store can be used to demonstrate exothermic reactions, and, if you’re interested, some organic chemistry.
You should be able to find all sorts of epoxies in the store, but the easiest to work with are the little cylindrical sticks that you have to massage with your fingers to mix the resin and hardener. As they combine, you can feel the epoxy getting warmer. The plumber’s epoxy warmed quite nicely.
You can also feel it get softer and easier to work, more malleable, so it could also be a good demonstration of plasticity. Especially since, as the chemical reaction occurs, the material hardens till, after a few minutes, it can’t be worked at all.
Epoxies are used a lot as adhesives and protective coatings, because they are extremely strong when they harden and can be quite sticky.
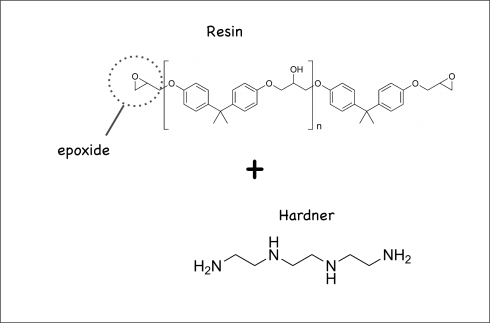
Their chemistry is fascinating. Epoxies work by mixing a resin and a hardener. The resin’s molecules have epoxide rings at either end, while the hardener’s molecules also have reactive ends. So when you mix them, they create long chained molecules called copolymers: polymers are long chains of a single molecule (the base molecule is called a monomer); copolymers are long chains with two base molecules instead of one.
The resulting network will not dissolve in any solvents, and resists all but the strongest chemical reagents. The plurality of OH groups provides hydrogen bonding, useful for adhesion to polar surfaces like glass, wood, etc.
–Robello (accessed 2011): Epoxy Polymers