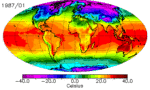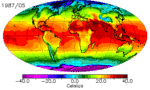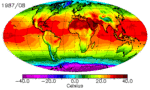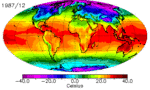
The continents heat up faster than the oceans, and they cool down faster too. You can see this quite clearly in the animation above: notice how cold North America gets in the winter compared to the North Atlantic. It’s why London has an average January low temperature of 2˚C while Winnepeg’s is closer to -20˚C, even though they’re at almost the same latitude. There are a few reasons for the land-ocean cooling differences, and they all have to do with how heat is absorbed and transported.
(1) Specific Heat Capacity. Water has a higher heat capacity than land. So it takes more heat to raise the temperature of one gram of water by one degree than it does to raise the temperature of land. 1 calorie of solar energy (any type of energy really) will warm one gram of water by 1 degree Celcius, while the same calorie would raise the temperature of a gram of granite by more than 5 degrees C. The Engineering Toolbox has specific heat capacities of common materials.
(2) Transparency. The heat absorbed by the ocean is spread out over a greater volume because the oceans are transparent (to some degree). Since light can penetrate the surface of the water the heat from the sun is dispersed over a greater depth.
(3) Evaporation. The oceans loose a lot of heat from evaporation. In the evaporative heat loss experiment, While there is some evaporation from wet soils and transpiration by plants, the land does not have anywhere near as much available moisture to cool it down.
(4) Currents. Not only do the oceans absorb heat over a greater depth, but they can also move that energy around with their currents. The solar energy absorbed at the equator gets transported towards the poles, while the colder polar water gets transported the other way. Currents help average out ocean temperatures.