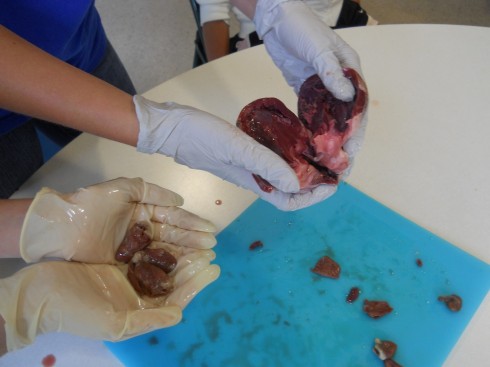
My middle school class has been looking at organ systems and we’ve started doing a few dissections. We compared chicken and pig hearts last week.
Pig hearts are large and four-chambered like ours, so they should have matched up very well with the diagrams from the textbook. However, real life tends to be messy, which is one of the first lessons of dissection. It was tricky finding all the chambers, and identifying the valves, even when you knew what you’re supposed to be looking for. It is especially difficult, as one of my students noted, because everything isn’t color coded.
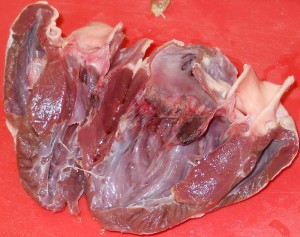
Chicken hearts are a bit trickier, because they’re a lot smaller. They also have four chambers, but the main chamber (the left ventricle) is so dominant that it’s easy to assume that there’s only the two (or even just one) chambers.
One student’s interesting observation was that the pig heart was a lot more pliable than the chicken heart. My best guess was that the chicken heart is made of a tougher muscle — it’s denser and more elastic (in that it rebounds to its original shape faster) — because has more work to do: chicken heart rates can get up to 400 beats per minute (Swinn-Hanlon, 1998) compared to 70 beats per minute for the pig.
To get a better feel for the texture, and to engage our other senses in our observations on the hearts, at the end of the dissection, which was conducted in the dining room using kitchen utensils, I fried up some of the chicken hearts with onions for lunch.
Notes
There are a number of nice labs for heart dissections online:
The hearts were purchased at the Chinese supermarket, Seafood City. There seems to be a greater organ selection on the weekends.