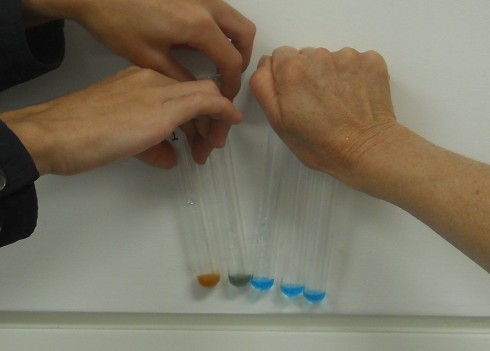
The standard biology-class test for simple sugars is to mix equal parts of Benedict’s solution and your sample solution, then heat it up in a hot water bath (80-100 ºC) for about five minutes. If there are simple sugars the mixture will turn from blueish green to reddish orange.
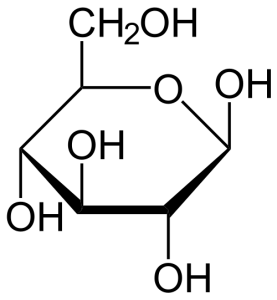
Simple sugars are those basic building blocks (monomers), which are chained together to form the more complex sugars and starches. The simplest are the monosaccharides (mono=one and saccharide=sugar) like glucose and fructose. Glucose is a chain or ring (see Figure 2) of six carbon molecules with the chemical formula C6H12O6. If you link two glucose molecules together, you get a disaccharide (di-two), which is called maltose.

Ms. Mertz did this experiment with her biology class last week using apple juice, oatmeal, corn syrup, honey, and an unknown as samples.

The biology class also tested for starch. Starches are really long chains of sugar molecules called polysaccharides. The simple sugar, Benedict’s solution test does not pick them up because the solution only reacts at the ends of the molecule, and with the long chains of the starch there aren’t that many sites for reactions.

The test for starch is to add a few drops of potassium iodine solution to your sample. Starch turns the resulting solution a bluish black.
