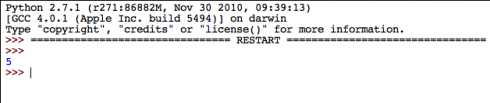
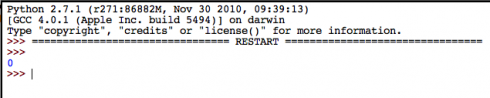
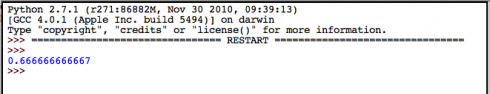
What could possibly go wrong?
— Kai Ryssdal (2011) in Freakonomics: Preventing a hurricane on Marketplace by American Public Radio.
In what one can only hope is an extremely tongue-in-cheek article, Marketplace discusses how we might geoengineer a solution to stop hurricanes forming.
Hurricanes get their energy from the warm surface waters in the tropics. The warm water evaporates, transferring heat from the oceans to the atmosphere as latent heat in the form of water vapor. As the air rises, the water vapor condenses to form water droplets (clouds) releasing the stored heat into the air, causing the air to rise faster, sucking up more moisture, and setting up a positive feedback loop that turns storms and hurricanes.
But they need a constant supply of warm water.
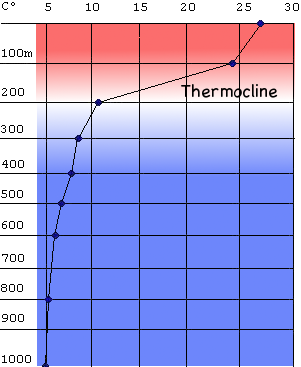
Unfortunately for the storms, the warm water in the tropics is only a thin layer, a couple of hundred meters deep, that sits above about 3,000 meters of colder deep-water. As the storms suck up the heat and moisture, they stir the oceans, cooling down the surface water, and leaving cooler water in their wakes. The cooler water means that subsequent storms have access to less energy.
The energy in the atmosphere and oceans “wants” to distribute itself evenly over the surface of the Earth. Hurricanes are just one violent means of moving heat from the tropics to the poles, and from the surface to the depths of the oceans.
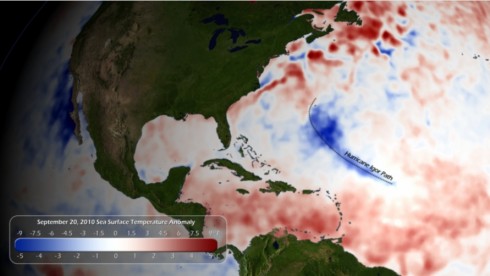
NOAA’s National Hurricane Center monitors sea-surface temperatures closely: it’s one of the key factors that go into their predictions of how bad the hurricane season is going to be, and what path a storm might take.
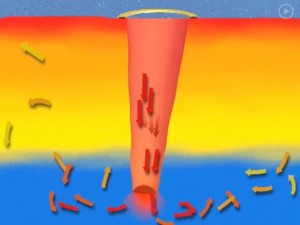
The suggestion in the Marketplace article is that we could build about 10,000 long tubes (called Salter Sinks) to connect the warm shallow surface water to the colder water below the thermocline. Wave energy at the surface would drive the warm water downward, causing mixing that would reduce the temperature of the surface water the storms feed off.
The devices might cost tens of millions of dollars per year, but that would be a lot less than the cost in property damage alone of a large storm like Irene, not to mention the loss of life it would prevent.
Apart from the “benign” environmental impact (according to Stephen Dubner) the only real question left is:
What could possibly go wrong?