This one’s for the nerds. It’s hard to argue against the elegance of using tau instead of pi. Natalie Wolchover explains, while Kevin Houston makes the argument in video:
Author: Lensyl Urbano
History of English in 10 Minutes Video
This is the first of an excellent series covering the history of English from The Open University. They make for a great spark-the-imagination lesson for etymology.
There’s lots more interesting videos at The Open University’s YouTube channel.
(via The Dish)
Malthusian Growth
I think I may fairly make two postulata.
First, That food is necessary to the existence of man.
Secondly, That the passion between the sexes is necessary and will remain nearly in its present state.
— Malthus (1798): An Essay on the Principle of Population via The Concise Encyclopedia of Economics
“Malthusian” is often used as a derogatory term to refer to alarmist predictions that we’re going to run out of some natural resource. I’m afraid I’ve used the term this way myself, however, according to Lauren Landsburg at the Concise Encyclopedia of Economics, Malthus is being unfairly maligned. He wasn’t actually predicting catastrophe but wondering why the catastrophes don’t usually happen.
What Thomas Malthus did, in 1798, was point out that while populations grow at a geometric rate – the U.S. population, he noticed, doubled every 25 years – but resources, like food, only increase at an arithmetic rate. As a result, any naturally growing population will eventually run out of resources.
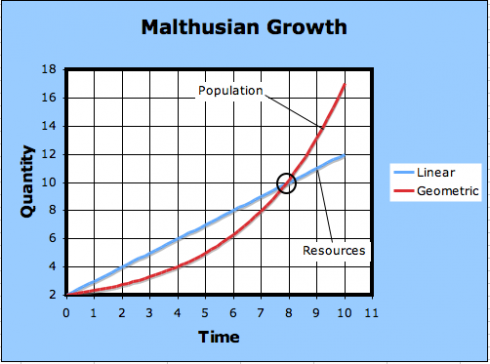
The linear equation has the form:
![]()
where y is the quantity produced, t is time (the independent variable), and m and b are constants. This should not look to unfamiliar to students who’ve had algebra.
The geometric equation is a little more complicated:
![]()
here a, g and c are constants. g is the most important, because it’s the growth rate – the higher g is the faster the curve will rise. You can play around with the coefficients and graph in this Excel spreadsheet .
At any rate, after the curves intersect, the needs of the population exceeds how much it can produce; this is the point of Malthusian catastrophe.
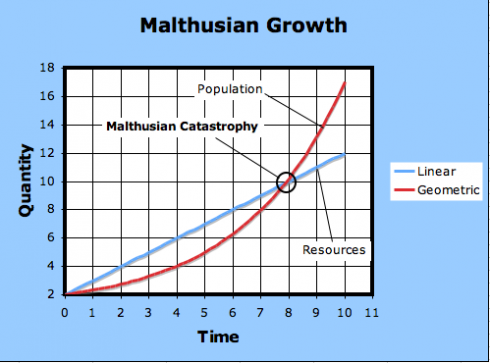
The observation is, indeed, so stark that it is still easy to lose sight of Malthus’s actual conclusion: that because humans have not all starved, economic choices must be at work, and it is the job of an economist to study those choices.
— Landsburg (2008): Thomas Robert Malthus from The Concise Encyclopedia of Economics.
The Modern Way to Draft a Constitution
Iceland’s drafting a new constitution. To make it more transparent and involve the citizenry, they made the draft available online and used social media, like Facebook, to get comments. The Constitutional Council even broadcast their weekly meetings on YouTube.
Suggestions from the public that have been added thus far include livestock protection and a clause that specifies who owns the country’s natural resources (the nation), …
— Kessler (2011): Iceland Croudsources Its Constitution
I’ve been having my students write their classroom constitution on our Wiki. It’s great for transparency and collaborative writing, but usually very few students are interested in looking beyond the section they write. The Iceland experiment is apparently running into a slightly different problem; well-wishers are clogging up the social media sites.
A Nefarious Application of Math

Cynical, but, if you consider the current “kinetic military action” in Libya, way to close to reality. Indeed, this highlights the question: When does it become too easy to go to war?
Jonathan Schell sums it up in the Guardian:
American planes are taking off, they are entering Libyan air space, they are locating targets, they are dropping bombs, and the bombs are killing and injuring people and destroying things. It is war. Some say it is a good war and some say it is a bad war, but surely it is a war.
Nonetheless, the Obama administration insists it is not a war. Why?
…, the balance of forces is so lopsided in favour of the United States that no Americans are dying or are threatened with dying. War is only war, it seems, when Americans are dying, when we die. When only they, the Libyans, die, it is something else …
— Schell (2011): Libya: it’s not a war if Americans can’t get hurt
Surviving the Anthropocene

65 million years ago, an asteroid hit the Earth just off the Yucatan Penninsula, kicking up enough dust in to the atmosphere (and perhaps setting off supervolcanos) to lead to the extinction of the dinosaurs. Geologists mark this mass extinction event as the end of the Cretaceous period and the beginning of the Tertiary; it’s called the K-T boundary. Paleontologists see a rapid change in the forms of life fossilized in the rocks above and below the boundary. The element iridium, which is relatively common on asteroids, but rare on Earth, can be found in a thin layer of the fallout from the asteroid impact all around the world.
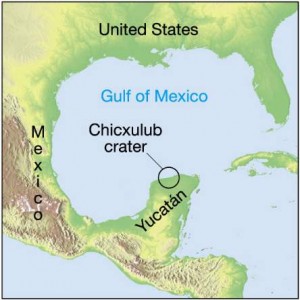
Well, the Earth’s going through another mass extinction event right now. In fact, even if humans were to go extinct right now, the remains of our cities and our impact on the global chemical cycles, will leave a distinct signature that geologists millions of years from now will be able to detect.
Geologists refer to the last 10,000 years, the period starting when the Earth warmed after the last glacial maximum, as the Holocene. This time period saw the emergence of agriculture, the rise of human civilization, cities, nuclear weapons, the internet. Now, given the enormous environmental changes we’re wrecking on the planet, some say we’ve entered a new geologic epoch that they’re calling the Anthropocene.
The question is: How long will it last?
Regardless of your philosophy, the recognition that we have entered a geologic age of humanity raises the obvious question of just how long such an age will last.
In the infamous KT boundary geologists can see evidence for a rather short-lived event that also reshaped the planet. Sixty five million years ago an asteroid struck the Earth, driving one of only five mass extinctions in the planet’s history. The loss of the dinosaurs turned out to be an opportunity for our mammal ancestors and led directly to our own age.
Since the Anthropocene appears to mark a sixth great extinction, one has to wonder what it will take for us to make it out of own era with civilization intact.
— Frank (2011): The Anthropocene: Can Humans Survive A Human Age?
2010 – Most Extreme Weather Since the Year Without a Summer
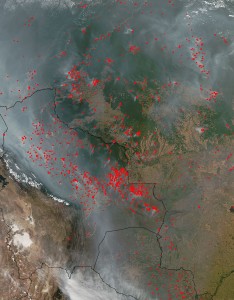
Jeff Masters has an impressively detailed post laying out the argument that 2010, with its record setting snowstorms, droughts, heatwaves, flooding, hurricanes, etc, had the most extreme weather since 1816, the year without a summer.
Looking back through the 1800s, which was a very cool period, I can’t find any years that had more exceptional global extremes in weather than 2010, until I reach 1816. That was the year of the devastating “Year Without a Summer”–caused by the massive climate-altering 1815 eruption of Indonesia’s Mt. Tambora, the largest volcanic eruption since at least 536 A.D. It is quite possible that 2010 was the most extreme weather year globally since 1816.
— Masters (2010): 2010 – 2011: Earth’s most extreme weather since 1816? on Weather Underground.

Notes
NOAA’s Historical Hurricane Tracks map is an excellent interactive webpage, and data source.
Resource Depletion: Overfishing
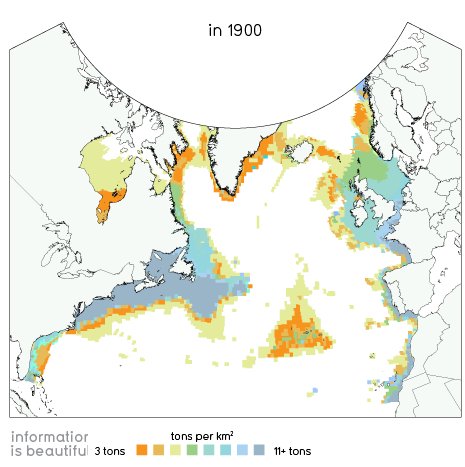
In 1900 fish stocks in the North Atlantic looked like this:
IN the year 2000, fish stocks looked like this:
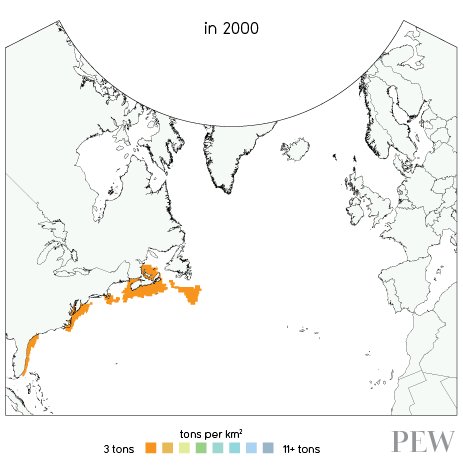
There are more data and visualizations on the European Ocean2012.eu site.

