
It was about 1.5 kilometers from the Research Lab to the estuary where we spent our first morning sampling (overview of the trip is here).

Walking along the beach to get there, we could see the beach houses to the right of us, across the narrow road of East Beach Drive, standing tall on columns to keep them above the reach of the storms. According to Stephanie, our guide, the storm surge from Hurricane Katrina in 2005, reached awfully close to the tops of the columns. The research lab, which is not elevated, lost an entire building to that hurricane. Indeed, much of the coast is still recovering from Katrina’s damage.
The white sandy beach, on the other hand, looked beautiful, which was a bit odd. After all, how did it survive the storm? Furthermore, when you think about it, this beach is located behind a string of barrier islands, which protect the coast from the full force of the waves coming out of the Gulf of Mexico, so how come there is enough wave energy to maintain a sandy beach. The relatively calm waters should allow finer grained sediment, like clay and silt, to settle out, and this area really should be a marsh. The answer, it seems, is that this is an artificial beach. Every few years, thousands of tons of sand are dumped along the coast to “replenish” the beachs.

This coastline really should be a tidal marsh, like the one we found when we got to the estuary. These Gulf-coast salt-marshes are fronted by a relatively short version of smooth cordgrass (spartina alterniflora), backed up by the taller, and more common black needlerush (Juncus roemerianus Scheele) .
Longshore Drift
Now, if this is a low-energy environment that allows silt and clay can settle out of the water column, where does the sand go so that it has to be replenished every so often? It is gradually moved along the coast by longshore drift.
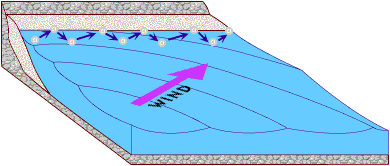
Waves hit the beach at an angle. As they break, the turbulent swash pushes sand up the beach at the same angle as the movement of the waves. As the wave retreats, the backwash, drawn by gravity, pulls sand perpendicularly down towards the water. The net effect, is that sand gradually moves down the coastline with each swash and backwash of the waves.
Since dumping tons of sand is expensive, engineers try other things to prevent the sand from running off down the beach. Someone, a very long time ago, had the great idea to build a wall sticking out from the beach to impede the sand in its unwanted migration. This type of wall is called a groin (or sometimes a groyne in polite company), and it does stop the sand. In fact, the sand builds up on the upwind side of the groin. Unfortunately, it does not stop the longshore drift on the downwind side, and that results in the erosion of a bay on that side.

Pufferfish
Beaches are also great places to find random things washing up. We lucked upon an unusually large pufferfish (family: tetraodontidae). It was quite puffed up. It was also quite dead.

Pufferfish are famous for being extremely poisonous. According to the National Geographic page on pufferfish, their tetrodotoxin over a thousand times more poisonous than cyanide, and there is no known antidote.

































