Paul Nicholas Worsey explains the physics and chemistry of how fireworks work. He’s cited in Sophie Bushwick great post compiling online resources that explain the science of fireworks.

Middle and High School … from a Montessori Point of View
Paul Nicholas Worsey explains the physics and chemistry of how fireworks work. He’s cited in Sophie Bushwick great post compiling online resources that explain the science of fireworks.

A little stick of epoxy from the hardware store can be used to demonstrate exothermic reactions, and, if you’re interested, some organic chemistry.
You should be able to find all sorts of epoxies in the store, but the easiest to work with are the little cylindrical sticks that you have to massage with your fingers to mix the resin and hardener. As they combine, you can feel the epoxy getting warmer. The plumber’s epoxy warmed quite nicely.
You can also feel it get softer and easier to work, more malleable, so it could also be a good demonstration of plasticity. Especially since, as the chemical reaction occurs, the material hardens till, after a few minutes, it can’t be worked at all.
Epoxies are used a lot as adhesives and protective coatings, because they are extremely strong when they harden and can be quite sticky.
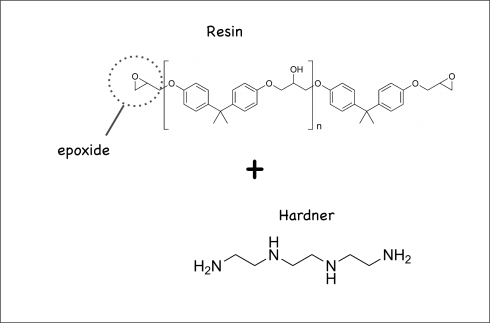
Their chemistry is fascinating. Epoxies work by mixing a resin and a hardener. The resin’s molecules have epoxide rings at either end, while the hardener’s molecules also have reactive ends. So when you mix them, they create long chained molecules called copolymers: polymers are long chains of a single molecule (the base molecule is called a monomer); copolymers are long chains with two base molecules instead of one.
The resulting network will not dissolve in any solvents, and resists all but the strongest chemical reagents. The plurality of OH groups provides hydrogen bonding, useful for adhesion to polar surfaces like glass, wood, etc.
–Robello (accessed 2011): Epoxy Polymers
The … organic materials in [some] meteorites probably originally formed in the interstellar medium and/or the solar protoplanetary disk, but was subsequently modified in the meteorites’ asteroidal parent bodies. … At least some molecules of prebiotic importance formed during the alteration.
Herd et al., 2011: Origin and Evolution of Prebiotic Organic Matter As Inferred from the Tagish Lake Meteorite
Amino acids are the building blocks of life as we know it. They can be formed from abiotic (non-biological) chemical reactions (in a jar with electricity for example). It’s been known for a while that amino acids can be found on comets and asteroids, but now this fascinating article suggests that a lot of the chemical reactions that created these precursors to life happened on the asteroids themselves. Then when the asteroids bombarded the Earth, the seeds of life were delivered. More details here.

This, of course, is just one of several hypotheses about the origin of life on Earth: livescience.com outlines seven.
Tiny quantities of dysprosium can make magnets in electric motors lighter by 90 percent, while terbium can help cut the electricity usage of lights by 80 percent.
–Lifton (2010): The Battle Over Rare Earth Metals
There has recently been a bit of a furor over the fact that, currently, China produces 90% of the world’s rare earth metals. Special properties of these elements are making them extremely important in a lot of high-tech and alternative energy technologies.
Fiber-optic cables can transmit signals over long distances because they incorporate periodically spaced lengths of erbium-doped fiber that function as laser amplifiers. Er is used in these laser repeaters, despite its high cost (~$700/kg), because it alone possesses the required optical properties.
–Haxel et al., 2005: Rare Earth Elements—Critical Resources for High Technology
The rare earths are so chemically similar that they’re lumped together in one corner of the periodic table, which is why they have not been used a lot until now. Only recently has their influence on elecromagnetic systems been discovered. Wikipedia has a good list of the elements with some of their uses.

Many people are worried about one country controlling so much of a single resource, especially since China cut its export quotas earlier this year. Fortunately, rare earth metals are found in places other than China, and, as the demand continues to outstrip supply, it’s just a matter of time for high prices to to bring more mining and recycling projects into production.

NPR’s Planet Money has a nice story on why gold is used for money. They take the entire periodic table of elements and eliminate the ones that don’t work because they’re too reactive, a gas, too common, or too toxic. You’re left with five precious metals, rhodium, palladium, silver, platinum and gold, but only one of them has a low enough melting temperature so that it can be worked easily and is not ridiculously rare.
Also, Tony Clayton has a wonderful webpage on Metals Used in Coins and Medals. It has some fascinating details about the history of these metals and their alloys in coinage. For example, “In Old English the Latin word aes was rendered as brass, thus the use of the word brass to mean money still found today, especially in Northern England. “
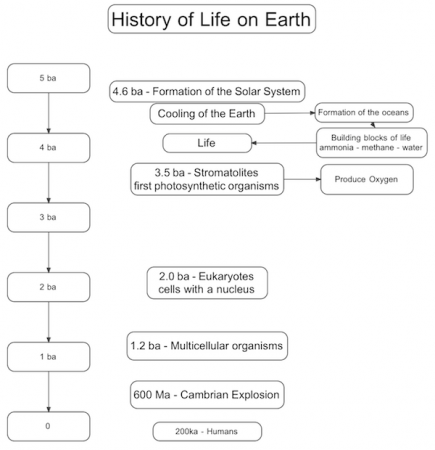
This year the theme is life. My central organizing structure is the timeline of life on Earth. I plan to link all of the discussions of taxonomy, phylogeny and genetics to this timeline over the course of the year.
The timeline above will be the first lesson. As with these things the trick is deciding how much detail to keep in and how much to keep out.
What I like is that it gives the general overview of when important things happen while leaving a lot of space for students to investigate. Most of what we’ll be seeing this year happened in the Cambrian and this timeline conveys that this is a very small part of the whole history of life. In fact, it’s only when we cover the biochemistry of genetics that we will be talking about the origins of life.
The website Exploring Life’s Origins has a great timeline. It also has some really neat sections, with very useful videos, on the formation of protocells and the origin of RNA on the early Earth that lead to life as we know it.
The one thing I left out that I’m still conflicted about is the Earth’s magnetic field. Recent research indicates it has been around since 3.2 billion years ago and its presence or absence may have had profound effects on life.

Having a magnetic field protects the Earth from the charged particles spewing out of the Sun, the solar wind. This makes life on land a lot easier since the solar wind’s particles are quite damaging to DNA. However, prior to the magnetic field forming all this damage to DNA may have also accelerated mutation and thus evolution.

On the last day of our trip we drove an hour west of St. Louis to Meramec Caverns. If you’re ever on I44 heading out of St. Louis you can’t miss it. From 30 km away you start seeing billboards, sometimes in pairs, almost every 100 meters.
Largely this is because it is a privately owned cave. Privately owned also means that they can do things to “enhance” the cave that you would not see at a National Park like Mammoth Cave. The light shows in certain caves were particularly interesting. Our tour guide was pretty good, entertaining and scientifically accurate for a general audience.

The presence of different colors in the rock formations (red, white and black) due to different metals in the carbonate precipitates could tie in very well with our discussion earlier this year of ionic bonding.
There are also historical tie-ins. The cave was the site of a skirmish during the civil war, because the bat guano was being used to produce gunpowder. Jesse James participated in that engagement and later used the cave as a hideout.

Finally, they have a reconstructed hut, which although it has nothing to do with the cave, has a bootlegger’s still does link with our discussion of steam distillation.

Why take a group of middle schoolers on a tour of the Anheuser-Busch factory? After all, no one will be trying any samples and the main point of the museum and the tour itself is to make people feel good about the company and buy more of its products. Which would be beer.

The answer is that the history of the company ties directly into the history of the industrial revolution in the U.S.. Its large scale industrial process was made possible by the serendipitous German immigration into St. Louis in the late 1800’s. Just then the expansion of grain farming in the mid-west and the railroads could supply the raw materials for beer on a massive scale. New assembly lines and automation could efficiently process these inputs, resulting in large scale production. Successes bred new inventions, with the company retaining the services of Rudolph Diesel to introduce his engine to the U.S..

The survival of the company during prohibition (1920-1933) was another point of interest. Anheuser-Busch survived by diversifying from its core business, making non-alcoholic drinks and selling baker’s yeast among other things. At the end of prohibition, Anheuser-Busch was the only large brewer left in the city St. Louis.

We also saw a bit of chemistry during the tour. The breakdown of complex starches into sugars as part of the fermentation process is a basic example of organic chemistry in action (polymers –> monomers). Light beer, for example, is kept in the tanks longer so that more of the starches are broken down into simple sugars. There were even a couple of big models of barley grains and hops buds showing their parts, that tie in to our life-science lessons next year.

Even the surfeit of advertising could be used to advantage; the first thing inside the door of the museum is a television playing Anheuser-Busch’s most successful commercials. We’ve been discussing propaganda all year, so the students were somewhat inoculated to the barrage of feel-good messages, and we specifically discussed this in our post-tour group meeting.
Our tour guides were great. Informative, friendly and willing to answer questions, I particularly appreciated that they joined us in our group discussion after the tour and answered the questions that came up as best they could.
This tour exceeded my expectations.