
We could have been talking about the nuclear meltdowns in Japan, but I’m not sure. Our conversations tend to wander. I remember trying to explain where the carbon atoms, that are so essential for life, came from. It’s been a while since we saw this topic, so I figured it wouldn’t hurt to go it over again. And then I found this wonderful image of the Tycho supernova from the Chandra space telescope. Supernovas are where the heaviest atoms are formed.
In the beginning … the big bang created just the smallest elements, hydrogen and helium. But even these tiny things have gravity, so they pull each other together until there’s so much stuff that the pressure at the center of the clump is enough to fuse hydrogen atoms together.
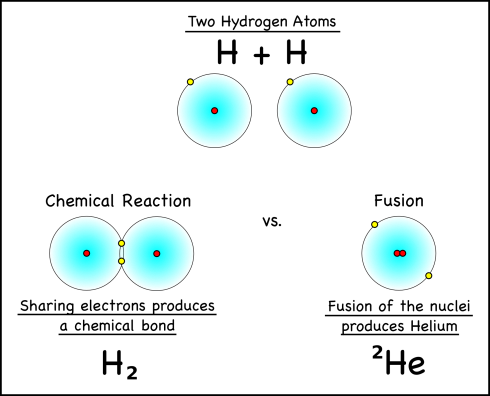
Now fusion is easy to confuse with chemical bonding that occurs around us every day. After all, the hydrogen in the atmosphere is usually in the form of H2, which is two hydrogen atoms bonding together by shared electrons.
With fusion, on the other hand, the single protons that make up the nuclei of the hydrogen atoms are pushed together to create a bigger atom, helium. I say pushed together, because it takes a lot of pressure to fuse atomic nuclei. And it also releases a lot of energy. Notice all that heat and radiation that comes from the Sun? All that energy was created by the fusion of hydrogen atoms; the smallest element, hydrogen, fuels the stars.
The huge amounts of energy released by fusion makes fusion power one of the holy grails of nuclear energy research. If we were able to create and control self-sustaining fusion reactions, just like what happens in the Sun, we would have a source of tremendous energy. There is a lot of research in this area. Some people have figured out how to build fusion reactors in their basements, but these use a lot more energy than they produce so they’re not very useful as a power plant (Barth, 2010). The ITER reactor, currently being built in France, aims to be the first to produce more electricity than it uses.
Now back to the stars. Hydrogen atoms fuse to form helium, but it takes a lot more pressure to create larger atoms: carbon has six protons, nitrogen seven, and oxygen eight. These elements are essential for life (as we know it). The only time stellar forces are great enough to produce these are when stars explode; an exploding star is said to have gone nova. Bigger atoms, like iron (26 protons), gold (79 protons), and uranium (92 protons) need even greater forces, forces that only occur when the largest stars go supernova.
So if these elements are only produced in novae and supernovae, how did they get to Earth? How did they get into your DNA?
Well when stars explode, a lot of these newly formed elements are blasted off into space. It’s a sort of cosmic dust. We could even call it stardust. It’s matter, just like the hydrogen and helium from the big bang, only bigger, which means they have more mass, which means they have more gravity.
The gravity pulls the stardust together with the hydrogen and helium sill floating around in space (there’s a lot of it), to form new stars, and, now that there are the larger elements to create them, rocks, asteroids, and planets.
So, if you think about it, some stars needed to have been formed, lived their lives (which consists of fusing hydrogen atoms until they run out), and exploded to create the matter that makes up the planets in our solar system and the calcium in our bones, the sodium in our blood, and the carbon in our DNA.
Notes:
1. Lots of information about Tycho’s Star on SolStation.com.