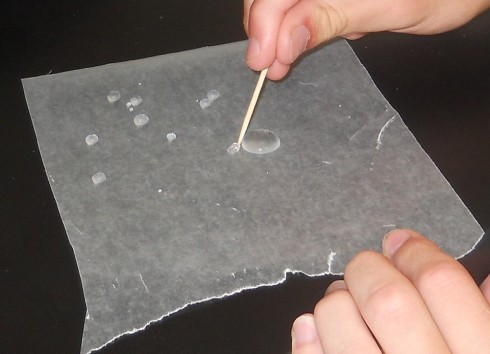
Ms. Mertz’s biology class chased water droplets around a piece of wax paper to study the properties of water. It was pretty neat how she had them name the droplets and then use a toothpick to drag them around, join them up, and split them apart.
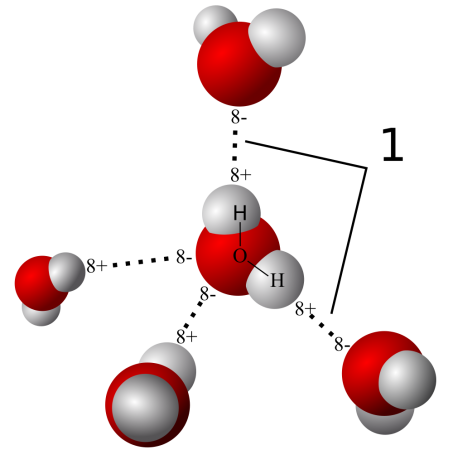
The water sticking together to form droplets is due to the hydrogen bonding between the water molecules: each water molecule has a slightly positively charged end and a slightly negatively charged end that causes molecules to stick together.
The ability to drag the water droplets around using a toothpick is because the cellulose fibers in the wood have their own slight charges that make them hydrophilic.
The students then tried dragging the water droplets around using a small piece of plastic straw, which was not supposed to be hydrophilic. However, it was a little hard to tell the difference between the straw and the wood. We’re not sure why, so we’ll have to revisit that part of the experiment again.
Ms. Mertz followed up with another nice little demonstration of the effect of soaps on water. She sprinkled some black pepper onto the surface of some water in a bowl, and then took a toothpick, dipped it into a bottle of liquid dish-washing soap, and then dipped the tip into the center of the bowl. The result was quite immediate, and quite dramatic.


The soap molecules are forced to form a thin layer on top of the water as their charged end is pulled down toward the water and their uncharged, hydrophobic end is pushed away.