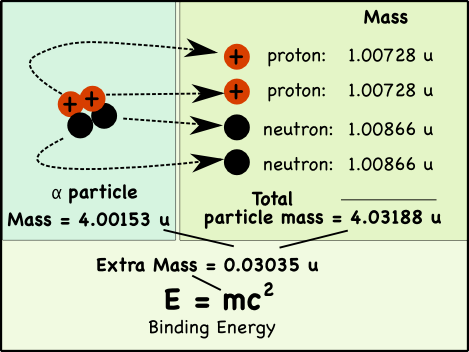
If you pull apart the nucleus of an atom, you’ll find that the mass of its parts is greater than the mass of the original nucleus. That extra mass is where nuclear energy comes from; it’s called the binding energy.
How so?

Take a helium atom for example. Its nucleus typically has two protons and two neutrons*, which in nuclear physics is usually called an alpha particle (α).
While we usually say that the mass of a proton is 1 atomic mass unit (u), its actually a little heavier. The mass of a proton is 1.00728 atomic mass units (u), while neutrons weigh 1.00866 u.
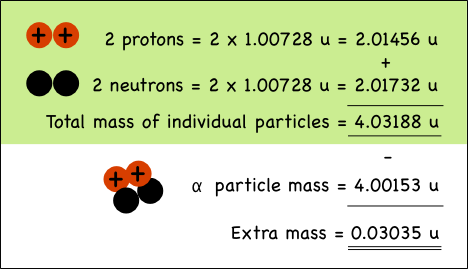
The combined mass of the two protons and two neutrons in the helium nucleus is 0.03035 atomic mass units more than the mass of a helium nucleus made up of the very same particles.
Why?
The one equation that everyone remembers from Einstein (perhaps from all the t-shirts) is:
![]()
Energy (E) is equal to mass (m) times some constant (c is the speed of light) squared. What it means is that mass is energy, and vice-versa.
When the four nucleons combine, the extra mass is transformed into the energy that holds them together in the nucleus of the atom. The mass can be directly converted to energy, the binding energy of the atom.
How much energy is released?
Somewhere around 10,000 times more energy is released from a single nuclear reaction compared to a single chemical reaction (like the combustion of TNT).
Footnotes
* Helium with two neutrons would be written ![]() , where the bottom number is the number of protons and the upper number is the atomic mass, which is the sum of the number of protons and the number of neutrons.
, where the bottom number is the number of protons and the upper number is the atomic mass, which is the sum of the number of protons and the number of neutrons.



