Why is the periodic table called the periodic table? Because of the periodic changes in the properties of the elements: there are patterns to the properties that repeat, time after time, as you go through the sequence of the elements. One key repetition, which affects the way different elements react, is in the electron configurations, however, other properties change as well. In fact, history of the periodic table
is a story of scientists trying to figure out the properties of unknown elements (not to mention figuring out that there were undiscovered elements) based on what they knew about the periodicity of the known elements.
File: periodic-table-properties.xls
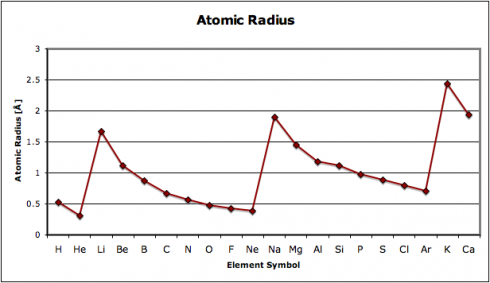
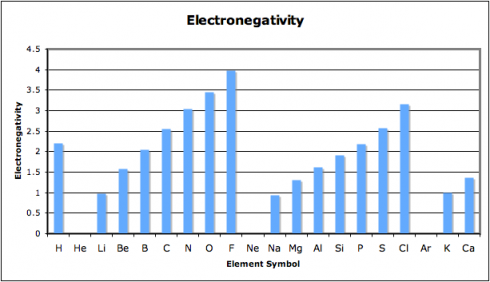
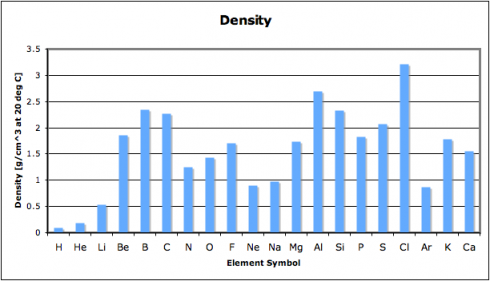
In this exercise, we look at four different properties that students need to be aware of: density, melting point, ionization energy, and electronegativity. I’ve compiled the data in this spreadsheet: periodic-table-properties.xls; and I handed out the first page, with the properties of the first 38 elements (periodic-table-properties.xls.pdf).
I broke the class into pairs and had each pair graph one of the four sets of data. With 16 students that meant that we had a replicate of each graph, so I could use the redundancy as a quick check that they’d done them correctly.
As they put drew their graphs I went around the classroom, paying special attention to the students working on ionization energy and electronegativity. Especially for the latter, I’d picked pairs who I figured would be able to get the graphs done quickly but would appreciate the extra challenge of figuring out what electronegativity actually is. This way, when everyone was done, the students could use their graphs to look for the patterns and explain what they’d found to the rest of the class.