This simple experiment was devised to estimate just how much heat is lost from a teacup due to evaporation as compared to the other types of heat loss (conduction and convection).
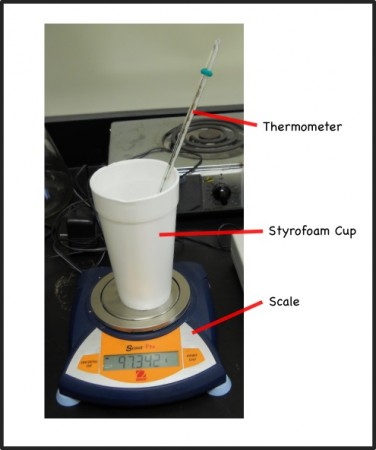
The idea is that if we can measure the mass of water that evaporates over a short period of time, we can calculate the evaporative heat loss because we know that the amount of heat it takes to evaporate one gram of water (its latent heat of evaporation) is 540 cal/g. So we’ll take some hot, almost boiling, water and weigh and take its temperature as it cools down.
Materials

It requires:
- A thermometer (Celcius up to 100 degrees)
- A styrofoam cup (because it’s light)
- A digital scale (to take quick measurements to tenths of a gram)
- A 100 ml graduated cylinder (optional)
- A beaker (100 ml) or cup that can go in the microwave
- water
Procedure
Our scale has a capacity of about 120 g so we need to make sure that the combined weight of our apparatus that will go on the scale is less. The plan is to have the styrofoam cup, with a thermometer and some water on the scale. Since we can be somewhat flexible with how much water is in the cup we’ll first weigh the cup and thermometer.
(my measurement, not necessarily yours)
Mass of styrofoam cup and thermometer = 29.6 g
So it should be safe to use 70 g of water, which is approximately equal to 70 ml since the density of water is 1 g/ml.
1. Measure the 70 ml of water in the graduated cylinder and put it into the beaker (or microwavable cup). The exact volume is not crucial here since we’ll be using the scale to measure the mass of water more precisely.
2. Microwave the water for about 40 seconds. Again you do not have to be too precise here, you just want the water to be close to boiling. The length of time you need to microwave the water will depend on the strength of your microwave. 40 seconds raised the temperature of my 70 ml of water from 22˚C to 82˚C. If you like you can calculate the heat absorbed by the water, and the effective power of the microwave from these numbers, but it is not necessary for this experiment.
3. Quickly place the hot water into the styrofoam cup with the thermometer on the scale and measure the mass and the temperature of the water.
4. Measure the mass and temperature of the water every 2 minutes for the next 10 minutes.
Calculations
1. Every time you took a measurement, the temperature and the mass should have dropped. The change in mass is due to evaporation. Every time one gram evaporated, 540 calories are lost. Calculate the amount of heat lost due to evaporation at every time measurement.
Hint: Evaporative Heat Loss = mass evaporated × latent heat of evaporation
QE = mE LE
2. Now that you know how much heat was lost, you can figure out how much of the temperature drop was caused by evaporation. Since the specific heat capacity of water is 1 cal/g/˚C, each calorie lost due to evaporation should have reduced the temperature of one gram of the water by one degree Celsius.
Hint: Evaporative Temperature Change = Evaporative Heat Loss × mass of water in container × specific heat capacity of water
∆TE = QE / (m Cw)
You should also the temperature drop caused by evaporation as a percentage of the total temperature drop. Hopefully, your result is less than 100%.
For comparison, here is my data: Evaporative Heat Loss Results and Calcualtions
Discussion and Conclusion
There are quite a number of things that might come up in discussion here, for example: just how large are the potential for measurement errors; and are the results comparable to an actual teacup.
My trial of this experiment indicated that about 69% of the heat loss was due to evaporation. It should be possible to also calculate the amount of heat loss from conduction through the walls of the cup; the thermal conductivity of styrofoam is 0.033 W/mK (via the Engineering Toolbox). The radiative heat loss can be estimated using Stefan’s Law, which can be used to account for all the different methods of heat loss.
Finally, there is no control described in this experiment. A useful thing to try would be to use a styrofoam cup with a lid.
Additional Notes
When my students tried this experiment they use a small (50ml) beaker and 25g of water. Their evaporative heat loss was only 44% of the total, probably due to the smaller volume of water, which as a larger surface-area to volume ratio, and the thinner, more conductive glass walls of the beaker.