The middle school class has been reading The Tempest for language arts this semester. However, I had not quite realized that it was a presentiment of for our outdoor education canoeing trip. And not metaphorically — the group worked amazingly well together — but there really was a massive storm while we were out paddling on the Current River.
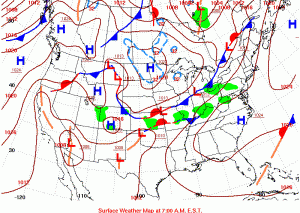
On Wednesday morning, two hours before dawn, a cold front heading south from Canada met a warm front coming north out of the Gulf. They met and stalled, pushing waves of clouds and thunderstorms over us from the west.
The first wave hit while we were in our tents; the second during breakfast. One student recounted that his highest point of the trip was when he tasted his first pancake that morning. His lowest point was when the pancake was promptly soaked with rain.
The third wave met us while we were in our canoes.
Searing lightning, flashing across the ridges of the valley. Blinding white. Immediate thunder, roaring straight through the ears, reaching in, taking the breath, grabbing at the soul. Drenching rain — cold and hard — a deluge. One of our guides described it, afterwards, as a religious experience. I think I know what she meant.

But the kids were awesome. Drenched, cold, and scared they paddled on. I was with a small group that was bringing up the rear. We were far enough behind that, for a long time, I could not tell how the students in the lead were responding. Especially when, at the height of the downpour, the lead group went around a bend in the river and out of our line of sight.

I knew they were with our lead guide (Leah), whose skill and competence had already been demonstrated earlier in the day when one of the canoes had flipped. Yet, one always worries about how kids will react in stressful situations. Following the current around a gravel eyot, however, I heard a loud cheer. There was the line of canoes, pulled over waiting for us. There were the students, soaked and perhaps a little bit relieved, but with no panic in the cacophony of voices.
When everyone had caught up, we continued on. Eventually, we hit a landing and called an end to the canoeing. Although the rain had stopped it was still cold. So, a few students decided that since the river water was so cold, if they waited in the water, when they came out they’d feel warm. “I’m willing to deceive my body,” they said.

While we waited for the bus, we talked a little about what we’d been through. Despite the stress — or perhaps because of — there was lots of laughter and a growing sense of camaraderie. I took the chance to highlight some of the quieter voices, those students who tend not to complain or be too excitable, and who took the time to appreciate the beauty and uniqueness of what they’d been through.
While I would not have planned it that way, the storm, our tempest, forged bonds of common experience that will resonate with this group for years to come.

Notes
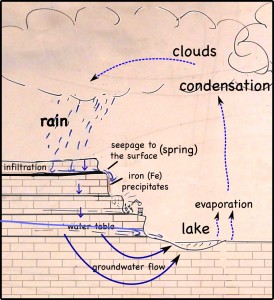
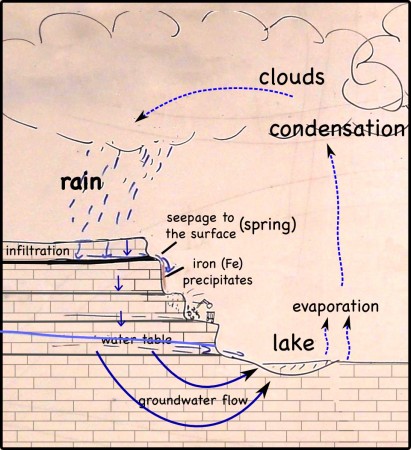
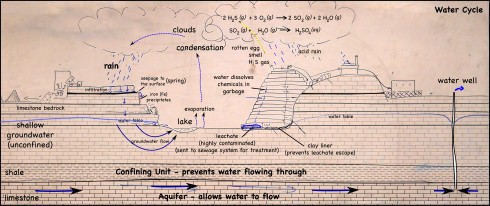
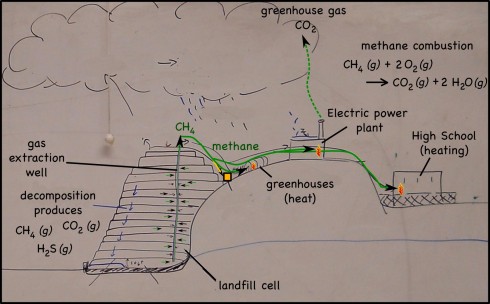
Infra-red satellite imagery from Wednesday, September 26th, 2012 shows the waves of thunderstorms passing over southern Missouri (yellow dot) very well.
The individual images come from NOAA’s GEOS archive: http://www.goes-arch.noaa.gov/
(From our Eminence Immersion)