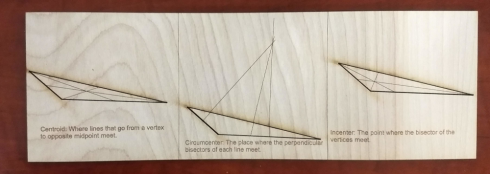
The area of a triangle is one half of the length of the base times the height:
![]()
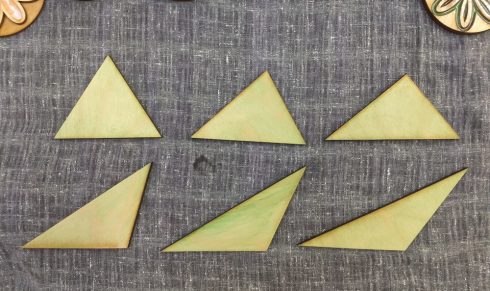
For my Geometry class, I made this set of six triangles to show that as long as the base and height are the same, all these triangles will have the same area.
Each student measured a triangle and found its area, which is a useful exercise in itself to get them to transfer the ideas and equations out of the book, and then the all compared their results. Their calculated areas were all within 5% of the actual value, which was not unexpected given that some small measurement error was inevitable.
Since you can use any side as the base, not everyone measured the equivalent side and height, so I had to demonstrate that similarity as I summed up the exercise.
For the next time I use this set, I’ve marked the one side that is 10 cm on each triangle for students to use as the base.