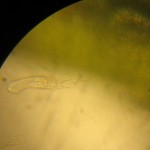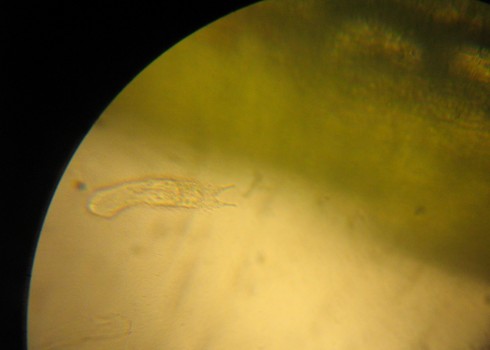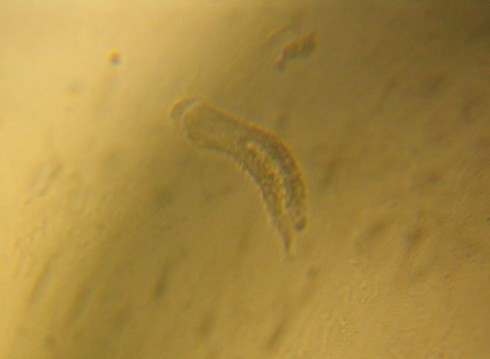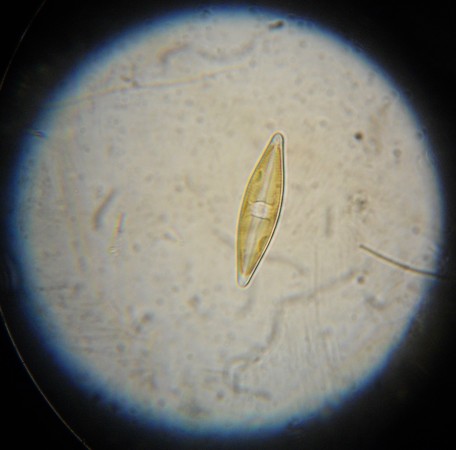
In addition to the basic stereoscopes with their fixed 10x and 30x magnifications, we also acquired a zoom stereoscope for more serious research projects. I tried it out with a sprig of lavender blossoms.

The clips on the stage weren’t particularly useful for holding something as small as a single flower, so, to see into the flower, I had to improvise with some of the dissection gear.

At larger magnifications, the focal depth is pretty small so it’s tricky trying to get the big picture. Even thought the camera didn’t quite capture it, you can make out the pollen grains.

I tried slicing the flower longitudinally to get a better look inside, and to see how difficult it would be to identify the major parts.

The photos turned out well using a point-and-shoot Nikon camera through the eyepiece, but even these pictures did not capture all the detail visible to the eye.

With the 2x objective attached, the microscope gets up to 90x magnification, but it becomes very hard clearly see anything after about 60x. All in all, the optics are good, and the lights bright enough to make for a very nice microscope.