
Although it was high in sulfur, the quarry company mined the thin coal seam that cut across the limestone quarry/landfill.
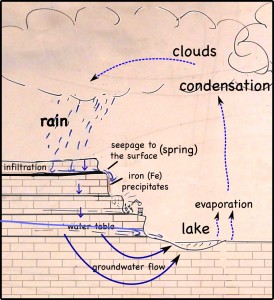
The layer of coal is pretty impervious to water, so it blocks vertical infiltration of water, which forces the water to the surface as springs.
At the surface, when the water is exposed to oxygen in the atmosphere, dissolved iron precipitates to produce a red mineral that stains the quarry walls.
The iron gets into the water when pyrite crystals (FeS2) in the coal dissolves. While the iron precipitates, the sulfur remains in the water, making it more acidic. Dealing with the acid can be a huge problem in large coal and metal mines.

Not all the pyrite is dissolved however, and since this particular coal seam has a lot of pyrite, it is not economical to burn since the burnt sulfur (as sulfur dioxide gas) would have to be captured — otherwise it produces acid rain.
