While we were talking about what I expect from lab reports, one of my students pointed out the Bibme website. It helps put together bibliography references in a variety of styles: MLA, APA, Chicago, and Turabian.
Middle and High School … from a Montessori Point of View
While we were talking about what I expect from lab reports, one of my students pointed out the Bibme website. It helps put together bibliography references in a variety of styles: MLA, APA, Chicago, and Turabian.

It’s spring, and what better time to study meiosis and dissect daffodils.

Daffodilusa (pdf) has nice description of how to dissect daffodils. However, I had students collect the flowers, and sketch the outsides and insides (longitudinal bisection) before I gave them the handout.
I wanted them to practice drawing diagrams and observing features first, before we got into the discussion of what the parts were and what they did, to make sure they’d not forgotten all they’d learned when we did our animal dissections last semester.
They laid out their grids, did some very nice drawings, and then labeled what they’d drawn, based on the handout, over the weekend.
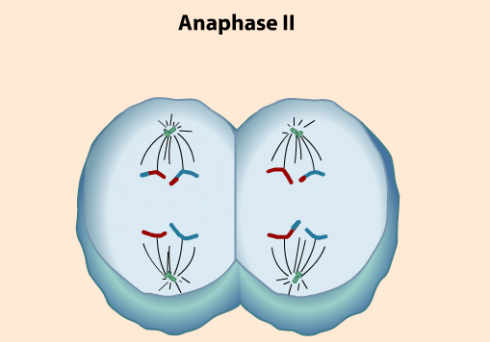
Going over meiosis in class today I used two videos. The first one was a bit simple. The second contained perhaps too much detail, but I showed it twice and stopped it at a few points on the second showing to point out the differences between mitosis and meiosis. I particularly wanted to highlight how genes are shuffled so the resulting reproductive cells have very different DNA from their parent. The shuffling is important because we’ll be comparing the advantages and disadvantages of asexual versus sexual reproduction later this week, as well as using Punnet squares to talk about heredity.
The first video:
The second:
Sulfur hexafluoride is transparent, so if you fill a fish tank with it you can’t really see that that tank’s filled with anything other than air. However, since sulfur hexafluoride is denser than air, you can float a light boat on the invisible gas for a cool demonstration of density.
Note: Air is about 80% nitrogen gas, which has the formula N2, and a molecular mass of 28 atomic mass units: the molecular mass is the sum of the atomic masses of all the atoms in a molecule. Sulfur hexaflouride has the formula SF6 and a molecular mass of 146 amu, making it about 5 times denser than air.
Steel is an alloy of iron and other elements in small amounts. The exact proportions of the small amounts of other elements can make the alloy stronger, more flexible, and/or more resistant to rusting among other things. Similar alloying is used to make aluminum stronger. You’ll often hear the saying, “Alloys are Stronger” (often used as an argument for more diversity). There is a lot of fascinating research and discoveries happening in the fields of metallurgical arts and sciences at the moment. However, YouTube user NurdRage demonstrates with some gallium and an aluminum can, alloys are not always stronger.
Smarter Every Day uses a high-speed camera to explain the rotational physics how cats manage to spin in the air and land on their feet.
More information on my other mitosis resources posts (including the mitosis dance).
Sumanas, Inc. has an excellent collection of biology-related videos, including good coverage of mitosis and meiosis.