A few of the steps along the Scale of the Universe flash app include the wavelengths of different colors of light. It’s a great way to show the show the relative sizes of these waves.
Middle and High School … from a Montessori Point of View

A few of the steps along the Scale of the Universe flash app include the wavelengths of different colors of light. It’s a great way to show the show the relative sizes of these waves.

In a modern variant on the Powers of Ten video, Cary and Michael Huang have created an excellent flash game that spans the scale of the universe, from the smallest, only hypothesized particles, through atomic, human, planetary, and galactic scales (to name a but a few), to the size of the universe itself. It goes further than the Cell Size and Scale flash app.
The link is here, but look out for an advertisement that takes up the game window, which will eventually let you through (or you can click the “Skip Ad” link on the bottom right of the ad).
A wonderful explanation of why scientists create computer models of the universe (and how it’s done); it’s a bit like the solar nebula model writ large.
(via The Dish).

When a photon of light hits an atom three things can happen: it can bounce off; it can pass through as if nothing had happened; or it be absorbed. Which one happens depends on the energy of the light, and which atom it is hitting. Hydrogen will absorb different energies from helium.
The interesting thing is that each atom will only absorb photons with exactly the right energy. You see, when the light hits the atom, the atom will only absorb it if it can use it to bump an electron up an electron shell.
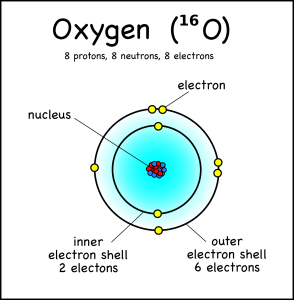
An oxygen atom, for example, has eight electrons, but these fit into two different electron shells. The innermost shell can only hold two electrons, so the other six go into the second shell (which can take a maximum of 8). This is the “ground state” of the atom, because it takes the least amount of energy to keep the electrons in place.
However, if the atom gets hit by just enough energy, one of the electrons can be bumped up into a higher shell. The atom will be “excited” and “want” the electron to drop back down to the lower shell.
And when the electron drops back, it will release the exact same amount of energy that it took to move it up a shell in the first place.
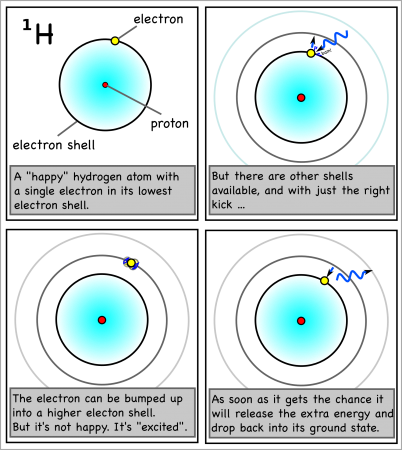
For hydrogen, the energy to bump up an electron from its first to its second electron shell comes only from light with a wavelength of 1216 x 10-10m (see here for how to calculate). This is in the ultraviolet range, which we can’t see with the naked eye.
However, the energy absorbed and released when the electron moves between the second and fourth shells is 6564 x 10-10m, which is in the visible range. In fact, it’s the red line on the right side of the emission spectrum shown at the top of the page.
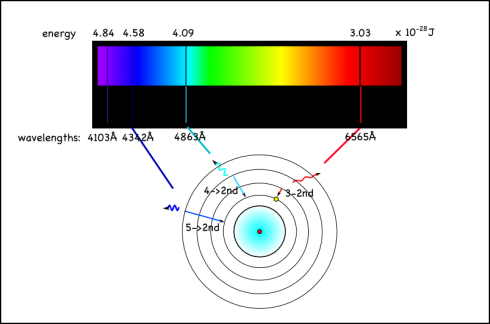
Since this emission signature is unique for each element, looking at the colors of other stars, or the tops of the atmospheres of other planets, is a good way of identifying the elements in them. You can also do flame tests to identify different elements.



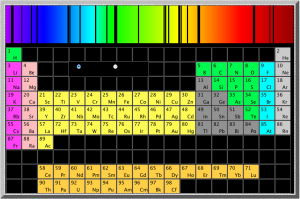
The University of Oregon has an excellent little Flash application that shows the absorption spectrum of most of the elements in the periodic table (NOTE that the wavelengths they give are in Angstroms (Å) which are 1×10-10m; or tenths of a nanometer (nm)).
The wavelength of light emitted for the movement of an electron between the electron shells of a hydrogen atom is given by the Rydberg formula:
where:
= wavelength of the light in a vacuum
= Rydberg constant (1.1×107 m-1)
and
are the electron shell numbers (the innermost shell is 1, the next shell is 2 etc.)
— see an example calculation here.
The amount of energy that corresponds to a particular wavelength of light is given by:
where:
= Energy (J),
= wavelength of the light in a vacuum (m),
= 6.6×10-34 Js,
= speed of light in a vacuum (3×108 m/s).
— via Wikipedia.
The National Institute of Standards and Technology (NIST) has more extensive emission spectra data.
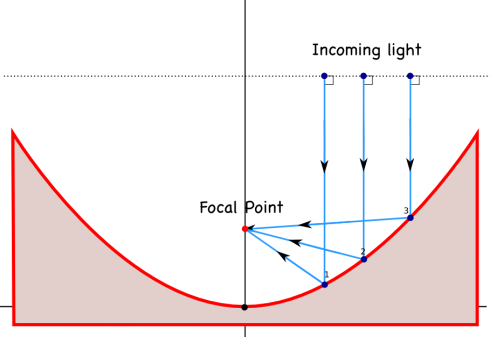
We’re talking about light and sound waves in physics at the moment, and NPR’s Morning Edition just had a great article on how the enormous, ultra-precise, mirrors that are used in large telescopes are made.
Astronomical observatories tend to use mirrors instead of lenses in their telescopes, largely because if you make lenses too big they tend to sag in the middle, while you can support a mirror all across the back, and because you have to make a lens perfect all the way through for it to work correctly, but only have to make one perfect surface for a parabolic mirror.
ScienceClarified has a great summary of the history of the Hubble Space telescope, that includes all the trouble NASA went through trying to fix it when they realized it was not quite perfect.

In addition, it’s interesting to note that you can also make a parabolic surface on a liquid by spinning it, resulting in liquid telescope mirrors .
Aerial robots are used to construct a tower. It’s pretty awesome, especially when you note that the robots don’t collide with each other, and plug themselves in when they realize they’re running out of power.
Robert Krulwich has more details on his NPR blog.
Just in time for our physics test — on electromagnetism — the Sun has had a Coronal Mass Ejection of charged particles that is heading toward the Earth.
[The Coronal Mass Ejection] is moving at almost 1,400 miles per second, and could reach Earth’s magnetosphere – the magnetic envelope that surrounds Earth — as early as tomorrow, Jan 24 at 9 AM ET (plus or minus 7 hours). This has the potential to provide good auroral displays, possibly at lower latitudes than normal.
— Fox, 2012: M8.7 Solar Flare and Earth Directed CME from NASA.
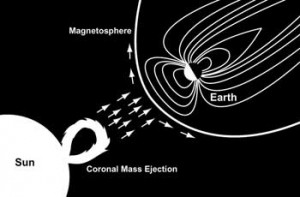
A Coronal Mass Ejection has about 100 billion tons of electrons, protons and other particles (NASA Cosmicopia, 2011), usually ionized, that would bombard the Earth and the atmosphere if we weren’t protected by the Earth’s magnetic field.
Most of the ions are deflected around the Earth but some get focused down toward the poles. At the poles, these ions hit nitrogen and oxygen molecules (that make up 98% of the atmosphere), exciting many of them. Excited atoms and molecules give off light. The light shows created are called the auroras.

I like the second video they post because, at the end, there is a splatter of interference from all the charged particles affecting the detector.

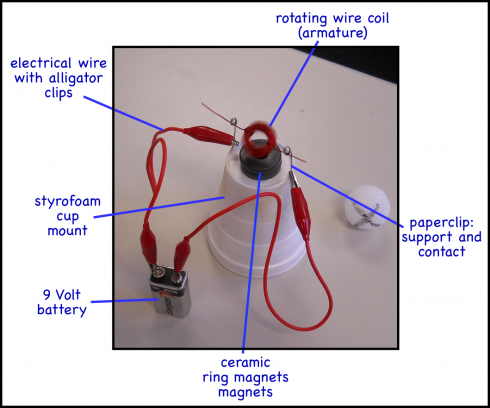
Our exercise in building simple electric motors was quite a success.
Students enjoyed doing it, even though it was challenging making the coil just right so it would spin easily. They persisted and enjoyed that wonderful eureka moment when it actually worked.
One group wanted to figure out how fast the armature was spinning. Because of small imperfections inherent to hand-made parts, we found that the armatures would bounce, ever so slightly, with each rotation. So the students recorded the sound using their laptop, and then counted rotations off the recorded sound wave. I think they came up with about 10 rotations per second.
I need to check if there’s a free phone app we can use that will show the sound waveform more efficiently — Pocket WavePad seems like it might work.
At the end, some students wanted to figure out just how fast they could get the motor running. Disdaining the online instructions, they went for more power, hooking up all the batteries they could scavenge from the other groups before I had to make them stop. They did manage to weld the insulating varnish on the coil wire to the paperclip contact before the end.
All the sparks did lead to a discussion of how arc welding works, however, which I was able to tie into the maths of transformers; cheaper arc welders (like this one) take in 20 Amps at 120 Volts, and output 70 Amps at 22 Volts.
This group did not want to stop, so I gave them permission to pass on P.E. for that one day, with a note that said they were doing some “remedial” physics. They got a kick out of that.