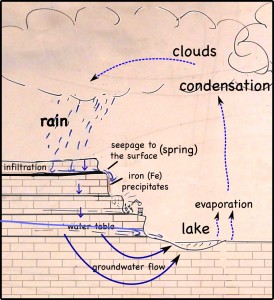
We took the middle and high school to see the Monet Water Lilies exhibit at the St. Louis Art Museum today. It was a nice tour; we saw some paintings, and we learned a little something about the impressionists.
One thought that occurred to me during an interesting conversation on the bus back to school, was how the development of abstract thinking skills affects our perception of the more abstract art. After all, it usually requires more effort to appreciate, understand and become affected a piece the more abstract it is. Which would suggest that art appreciation would be useful practice for adolescents who are honing their higher-level cognitive skills.
The tour also left me with one unanswered question, however: are we seeing fog or smog in Monet’s painting of the Charing Cross Bridge in London.

London is famous for its fogs, but this painting was done in 1899, well into the industrial revolution, and the yellow tints suggest a pea-souper.