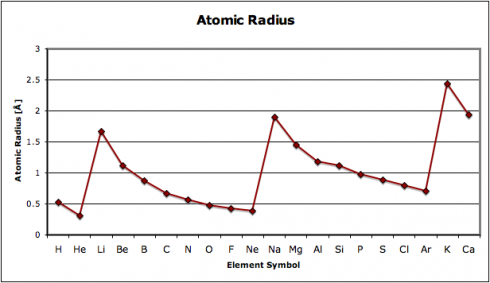
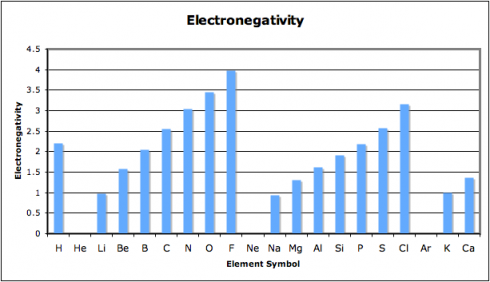
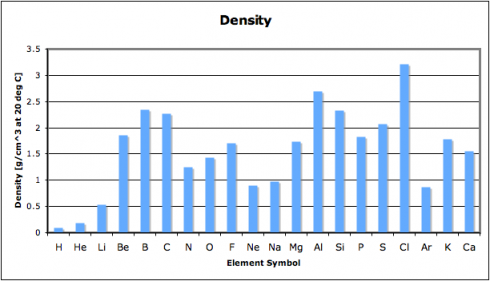
… observed astrophysical black holes may be Einstein–Rosen bridges, each with a new universe inside that formed simultaneously with the black hole. Accordingly, our own Universe may be the interior of a black hole existing inside another universe.
— Poplawski, 2012: Radial motion into an Einstein–Rosen bridge (pdf) in Physics Letters B.
For some reason the Big Bang theory came up during a middle-school class discussion last week; specifically, the mind-bending question of what exactly was there just before and at the beginning of the universe. We also meandered into the question about what’s at the edge of the universe — and how can the universe have an edge where time and space end.
There really aren’t any satisfactory answers to these questions, especially not for middle schoolers. But it allowed me to talk about how science is really just the best explanations for the known observations. Unsatisfactory answers to these questions are why we have the scientific method.

To throw a little more mind-bending fuel on the fire, however, I’m going to show the class the article quoted above.
It’s an interesting example of how scientists see the universe through math. And how they strive to make sense of things they can’t see or touch.