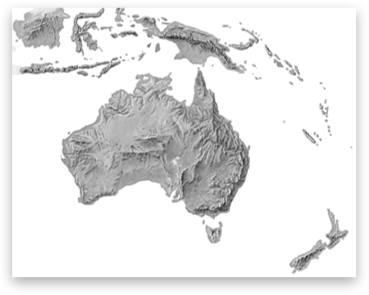
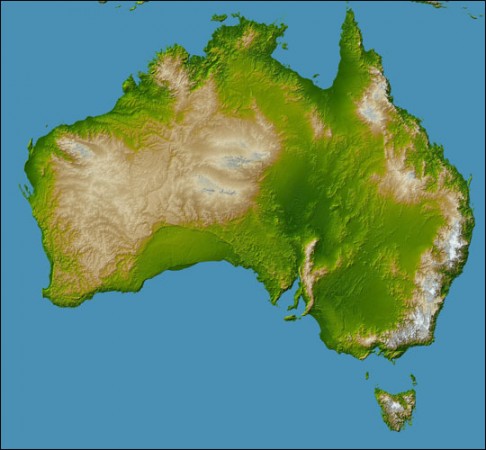
The Shaded Relief Archive is a great source of continental scale shaded relief maps. Dr. A. used them when the middle-schoolers built their 3d models of Australia and Antarctica for geography.

NASA’s Earth Observatory is another great source.
Middle and High School … from a Montessori Point of View

The Shaded Relief Archive is a great source of continental scale shaded relief maps. Dr. A. used them when the middle-schoolers built their 3d models of Australia and Antarctica for geography.

NASA’s Earth Observatory is another great source.

Andrew Sullivan pulls together commentary on a recent research paper that shows that the costs of waiting to act on climate change, far outweigh the costs of acting now. The longer we wait, the more it’s going to cost to prevent the most dangerous effects of climate change. Unfortunately, the costs of waiting will be paid in the future (as will the benefits), so there’s less motivation to act now.
Two degrees is the level that is currently supported by over 190 countries as a limit to avoid dangerous climate change …
“Ultimately, the geophysical laws of the Earth system and its uncertainties dictate what global temperature rise to expect,” said Rogelj. “If we delay for twenty years, the likelihood of limiting temperature rise to two degrees becomes so small that the geophysical uncertainties don’t play a role anymore.”
–Climate Progress (2013): Nature: Limiting Climate Change Will Become Much Harder ‘And More Expensive If Action Is Not Taken Soon’ on ThinkProgress.
On top of this, Fiona Harvey reports on an International Energy Agency report that suggests:
The world is likely to build so many fossil-fuelled power stations, energy-guzzling factories and inefficient buildings in the next five years that it will become impossible to hold global warming to safe levels, and the last chance of combating dangerous climate change will be “lost for ever”, according to the most thorough analysis yet of world energy infrastructure.
— Harvey (2011): World headed for irreversible climate change in five years, IEA warns in The Guardian.
Both [thermometer and proxy] records also show that the global warming in the last 15 years of the record (1980–1995) is significantly faster than that of the long-term trend (1880–1995).
— NOAA (2013): Independent Evidence Confirms Global Warming in Instrument Record.
To figure out what the weather and climate were like in the past, before things like thermometers were invented, scientists use proxies such as: the change in tree ring thickness; differences in the isotopic composition of shells and rocks; records of species change in the oceans; gases in bubbles trapped in glacial ice (as well as the character of the ice itself). Paleoclimatologists at NOAA have analyzed 173 different proxy records to provide a lot more evidence that the increase in temperature we’ve been measuring for the last 150 years (with thermometers) is real.
<a href=”http://www.curatorscode.org” target=”_blank” style=”font-family:sans-serif;text-decoration:none” >↬</a> The Dish

Fully loaded, the first stage of the Saturn V rockets that launched the Apollo missions would burn through a liter of fuel for every four centimeters it moved. That’s 5 inches/gallon, which, for comparison, is a lot less than your modern automobile that typically gets over 20 miles/gallon.
Covalent bonding happens when atoms share electrons, unlike with ionic bonding where one atom gives electrons to another.
Why do some combinations of atoms make ionic bonds and others covalent bonds? The answer has to do with electronegativity, which is the ability of atoms to attract electrons to themselves. Atoms that have similar abilities to attract electrons to themselves will likely form covalent bonds.
For either type of bond, the atoms have the same objective. All atoms “want” filled outer electron shells. When sodium reacts with chlorine for example, sodium has one electron in its outer shell and chlorine is one short of a filled outer shell so it’s “easiest” for sodium to just donate its electron to chlorine to make them both happy.
However, when two similar atoms bond it’s often easier to share electrons.
Consider two hydrogens bonding covalently to form hydrogen gas (note: help on drawing atoms).
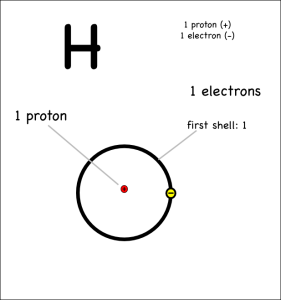
Each hydrogen has only one electron, and they both pull equally at the electrons so neither can give their electron away or take the other’s electron. Instead they share.
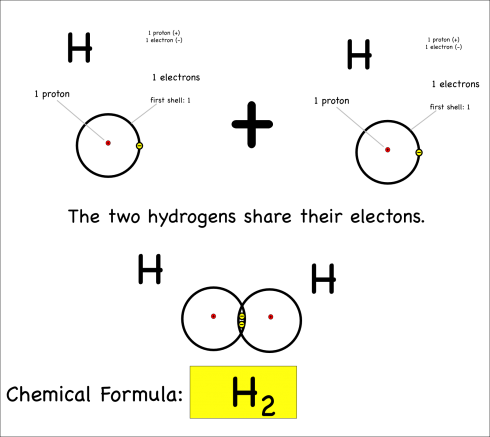
By sharing, they now each have two electrons in their outer shell, which is now full (since it’s the first shell), and both atoms are happy. This is covalent bonding.
The chemical reaction could be written as:
H + H –> H2
Now consider what happens when hydrogen atoms bond with oxygens. Oxygen atoms have 6 electrons in their outer shells, but they would like to have 8.
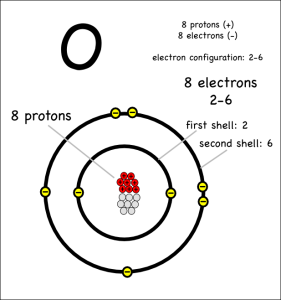
Oxygen atoms aren’t strong enough to take away the hydrogen electrons, so they share with covalent bonds. Each oxygen has to react with two hydrogens to get the two extra electrons it needs to end up with 8 electrons in its outer shell.
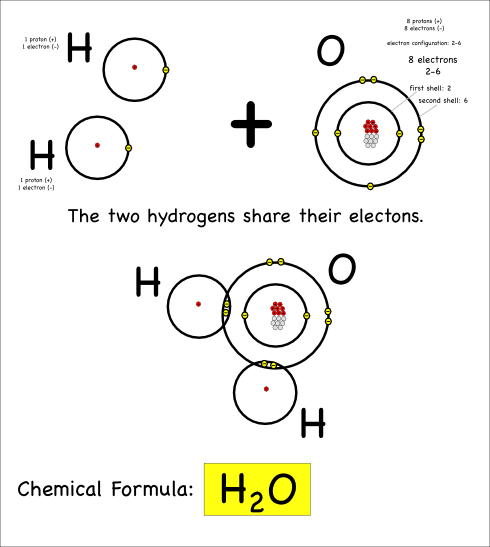
Thus we create water, which has the chemical formula H2O, and the chemical reaction can be written:
2 H + O –> H2O
Covalent molecules can be large and complex, in fact, one strand of your DNA will have somewhere around a billion atoms.
To make these easier to draw, you can represent each element by its symbol and each bond by a line. Remember, each covalent bond represents a pair of electrons that are shared.
So our water molecule would be drawn like this:
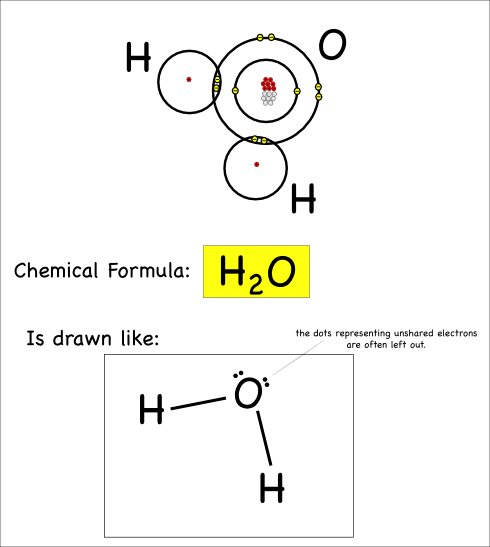
This is called a Lewis Dot structure. In addition to the lines showing the bonds, you’ll notice the dots that show the unbonded electrons: these dots are usually paired up.
The last thing I’ll point out here is that atoms can share more than just one pair of electrons. When they share four electrons that means there are two bonds, which is referred to as a double bond.
Oxygen atoms bond with each other like this to make the oxygen gas we breathe.
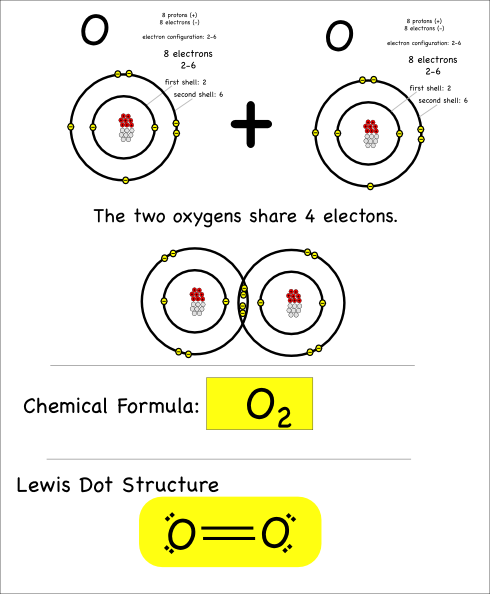
Now you can try drawing these covalent molecules:
Now that we’ve learned how to draw individual atoms (and have an online reference for the first 20 elements), let’s consider ionic bonding.
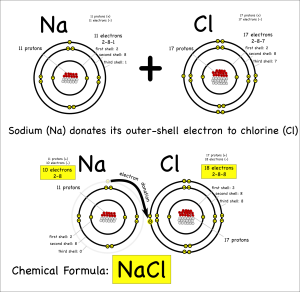
The key thing to remember is that atoms all “want” to have their outer electron shells filled. So while a sodium (symbol: Na) atom is happy* enough that it has the same number of protons and electrons (11 each) it could be happier if it got rid of the extra electron in it’s outer shell.
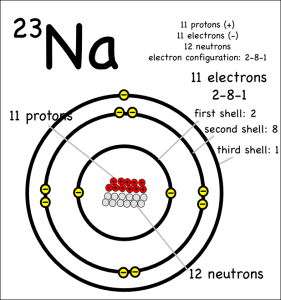
It can get rid of the electron by donating it to another atom that would be happier with an extra eletron. Something like chlorine (symbol: Cl) that only has 7 electrons in its outer shell, but wants to have 8.
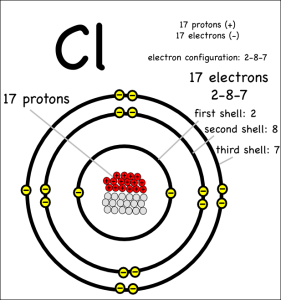
When one atom donates electrons to other atoms this creates a bond called an ionic bond. The molecule created is called an ionic molecule. In this case, sodium and chloride react to produce sodium chloride (chemical formula: NaCl).
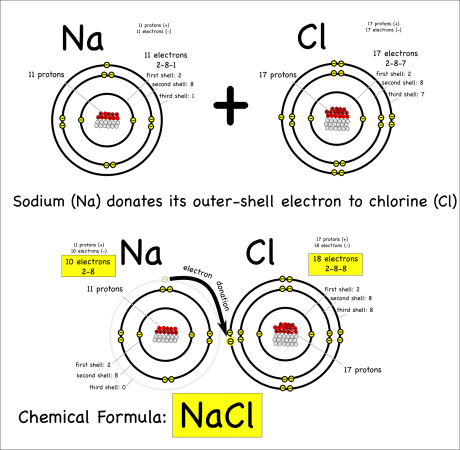
The chemical reaction can be written as:
Na + Cl –> NaCl
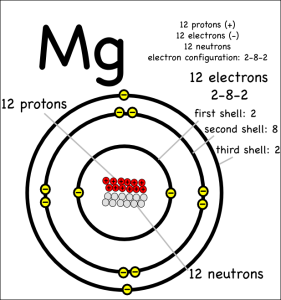
Now consider what happens when magnesium (symbol: Mg) reacts with chlorine.
Magnesium has two electrons in its outer shell that it wants to get rid of.
A single chlorine atom can’t take both, since chlorine only needs one electron to fill its outer electron shell. However, magnesium can give one electron to two different chlorine atoms to create a molecule with three atoms total.
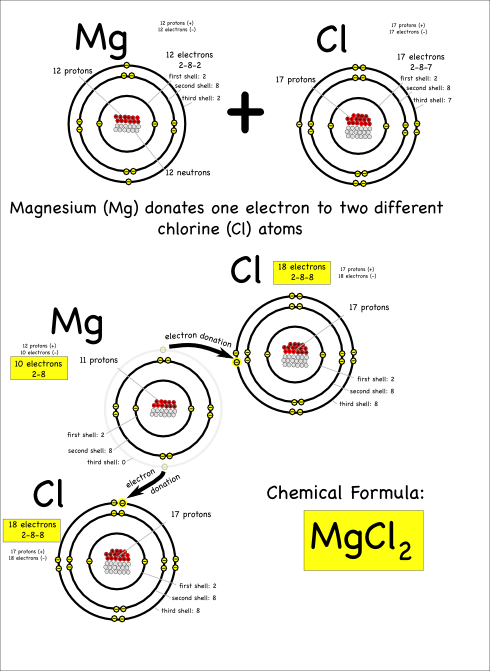
The resulting compound is called magnesium chloride, and is written as MgCl2. The subscripted 2 indicates that there are two chlorine atoms in each magnesium chloride molecule.
The chemical reaction can be written as:
Mg + 2 Cl –> MgCl2
Notice that each magnesium atom reacts with two chlorine atoms (Mg + 2 Cl) to produce a compound with one magnesium and two chlorines bonded together (chemical formula: MgCl2).
Now, for homework, you can try figuring out what is the chemical formula for the following ionic compounds:
Be sure to:
Good luck. Next we’ll try covalent bonding.
* Think of happiness as energy. Like people, atoms are happier to be in low energy states.
When we looked at the patterns in the periodic table, one of the things I had my student graph was the electronegativity. Electronegativity is the ability of atoms to attract electrons to themselves. You’ll note that chlorine’s electronegativity is high, while sodium’s is low.
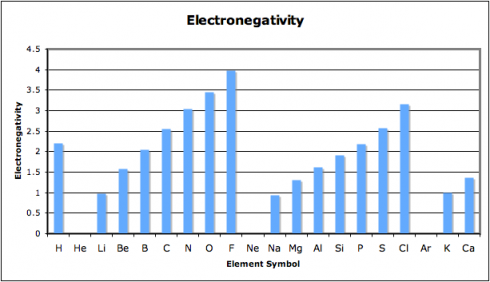
So chlorine will attract electrons to itself strongly, while sodium will not. This is why (more or less) sodium will end up donating its electron and why chlorine is happy to accept it.
When atoms with a large difference in electronegative bond together, the bonds tend to be ionic.

Our school was recycling some old computers, so my students convinced me that it would be worthwhile o dissect a few of them to see if there was anything worth saving. It was quite remarkable to see just how interested they were in examining the insides of the machines — a few desktop computers and a monitor — but I guess I shouldn’t have been surprised. After all, it’s getting harder and harder to open up their iPods and other electronics, and even more difficult to repair and repurpose them, so I can see why students would jump at the chance of looking inside a device. Also, they tend to like to break things.

To get them to think a little more about what they were seeing, I got a couple students to draw a scale diagram of one of the motherboards, and write up a report on what they’d done.

Some of the other students spent their time trying to make all the motors, LED’s, and lasers work by hooking them up to 9-Volt batteries. Then they found the fans… and someone had the brilliant idea that they could use it to make a hovercraft. Using a gallon sized ziplock bag and some red duct tape, a prototype was constructed.
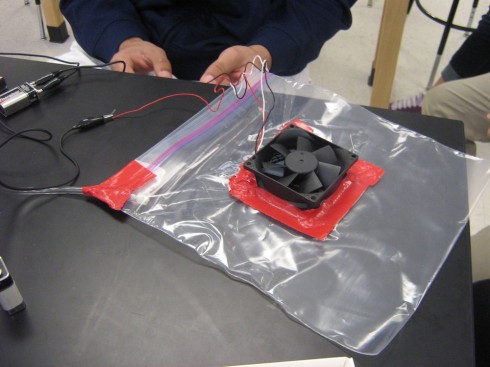
The fan would inflate the bag which would then let air out the bottom through small holes. I convinced them to try to quantify the effectiveness of their fans before they put the holes in by hooking the bag up to one of our Vernier pressure sensors that plug into their calculators. Unfortunately, the sensor was not quite sensitive enough.

This was not how I had planned spending those days during the interim, but the pull of following the students’ interests was just too strong.

We took a school trip to the ski slopes in Hidden Valley. It was the interim, and it was a day dedicated to taking a break. However, it would have been a great place to talk about gradients, changes in slopes, and first and second differentials. The physics of mass, acceleration, and friction would have been interesting topics as well.

This year has been cooler than last year, but they’ve still struggled a bit to keep snow on the slopes. They make the snow on colder nights, and hope it lasts during the warmer spells. The thermodynamics of ice formation would fit in nicely into physics and discussion of weather, while the impact of a warming climate on the economy is a topic we’ve broached in environmental science already.
