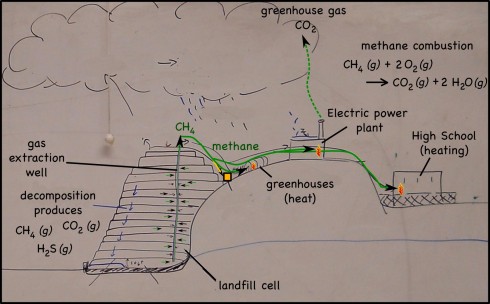
Decomposing waste in landfills produces quite a lot of methane gas (CH4). Perhaps better known as natural gas, methane is one of the simplest hydrocarbons, and a serious atmospheric pollutant (it’s a powerful greenhouse gas). In the past the methane produced was either released into the atmosphere or just burned off.

I remember seeing the offshore oil rigs burning natural gas all night long — multiple miniature sunrises on the horizon — in the days before the oil companies realized they could capture the gas and sell it or burn it to produce energy. The landfill companies have realized the same thing. So now, wells pockmark modern landfills and the methane is captured and used.

First, of course, the hydrogen sulfide gas (H2S), is separated from the methane — H2S produces acid rain, so it’s emissions are limited by the EPA — then, the gas from the landfill we visited, is piped to:
- greenhouses, where it’s burnt to produce heat;
- the Pattonville High School, which is right next to the landfill and burns the gas for heating;
- and (soon) to a electricity generating power plant that will burn the gas to produce heat which will boil the water that will produce the steam that will turn the turbines that will generate the electricity.

You may have noticed the common theme of all these uses of natural gas: it has to be burned to be useful. The combustion reaction is:
CH4 (g) + 2 O2 (g) —-> CO2 (g) + 2 H2O (g)
which produces carbon dioxide (CO2) that is also a greenhouse gas, but is, at least, not nearly as powerful at greenhouse warming as is methane.