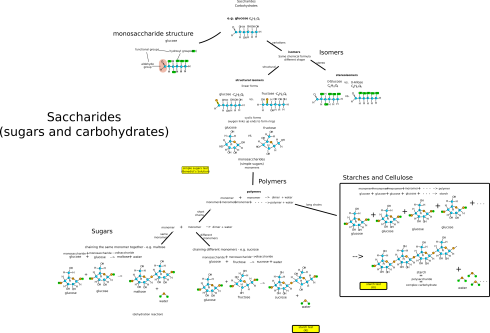
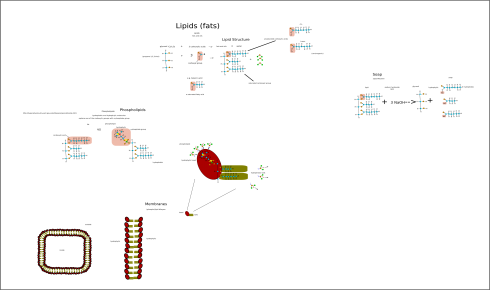
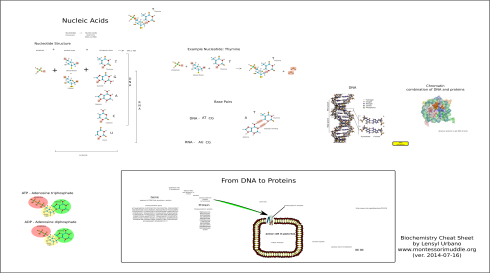
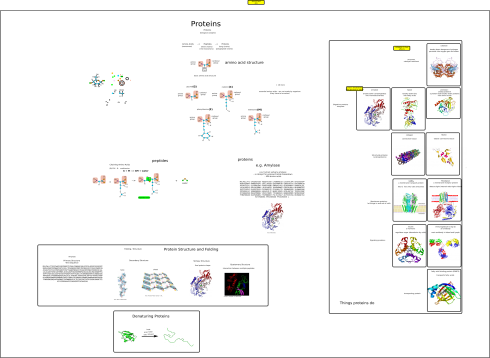
My notes on the four macromolecules that are essential to life as we know it: proteins, fats (lipids), carbohydrates (saccharides), and nucleic acids.
Middle and High School … from a Montessori Point of View
MolView is a great site for drawing molecules and rendering them in 3D.
Scientific American has an article on “The Scientific Secret of Fluffy Pancakes” that nicely covers the chemistry of gluten. The article includes a recipe, but I tend to prefer buttermilk pancakes (Cook’s Illustrated has a great buttermilk pancake recipe that I find tastes even better if you leave out the sugar).
My wife has been experimenting with gluten-free pancakes and I’ve discovered that I like hers even better because of all the ground nuts it includes. However, in the gluten-free recipes, crucially, Xanthan gum is used to replace the gluten in capturing the carbon dioxide bubbles created by the baking powder reacting with the acid in the recipe. While the gluten is sensitive to how much the batter is mixed (stretching out the proteins), the quantity of Xanthan gum is more important in the gluten-free recipes.
↬ Maggie Eisenberger.
Ms. Wilson’s chemistry class is looking at basic chemical reactions, and today they got to fire an acetylene cannon. When calcium carbide (CaC2) reacts with water (H2O) they produce acetylene (C2H2), which is quite explosive.
CaC2 + 2 H2O → C2H2 + Ca(OH)2
Acetylene is so flammable, because its carbons are held together by a triple bond: when the triple bond breaks it releases a lot of energy (about 839 kJ per mole).
Table 1: Bond strengths of simple hydrocarbons with carbon to carbon bonds
| Name | Chemical Formula | Diagram | Carbon to Carbon Bond Strength (kJ/mol) |
|---|---|---|---|
| Acetylene | C2H2 |  |
839 |
| Ethene | C2H4 |  |
611 |
| Ethane | C2H6 |  |
347 |
The explosion is a result of the combustion of the acetylene:
2C2H2 + 5O2 –> 2H2O + 4CO2
And this whole process — carbide plus water to give acetylene, which is then burned — was used by miners in the early 20th century to make headlamps (among other types of lamps).

The cannon itself is a simple device, made of a 50cm tube of 2-3 inch diameter PVC (sorry about the mixed units), with a screw cap at one end. The carbide grains (about 0.5 g) are placed on the inside of the cap, which is then screwed on to the bottom of the tube. A few drops of water are then added through a small hole in the PVC using a plastic dropper — you can listen for the sizzling to tell if the carbide decomposition reaction is happening. Finally a flame is applied to the same hole as the water. The sock, by the way, is just lightly tucked in near the top of the PVC tube, about 5 cm in.
The explosion was loud, and Ms. Wilson’s sock traveled about 10 meters. It was suitably impressive. I think the student who was the most impressed was the one who had weighed out the calcium carbide, becaues 0.5 grams is really only four or five grains.

A little stick of epoxy from the hardware store can be used to demonstrate exothermic reactions, and, if you’re interested, some organic chemistry.
You should be able to find all sorts of epoxies in the store, but the easiest to work with are the little cylindrical sticks that you have to massage with your fingers to mix the resin and hardener. As they combine, you can feel the epoxy getting warmer. The plumber’s epoxy warmed quite nicely.
You can also feel it get softer and easier to work, more malleable, so it could also be a good demonstration of plasticity. Especially since, as the chemical reaction occurs, the material hardens till, after a few minutes, it can’t be worked at all.
Epoxies are used a lot as adhesives and protective coatings, because they are extremely strong when they harden and can be quite sticky.
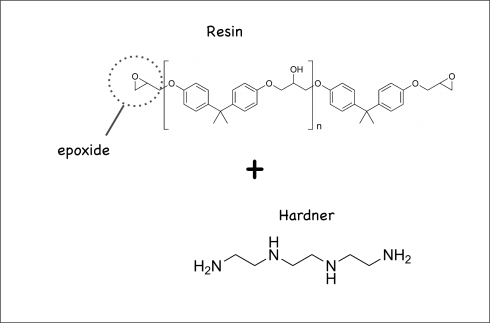
Their chemistry is fascinating. Epoxies work by mixing a resin and a hardener. The resin’s molecules have epoxide rings at either end, while the hardener’s molecules also have reactive ends. So when you mix them, they create long chained molecules called copolymers: polymers are long chains of a single molecule (the base molecule is called a monomer); copolymers are long chains with two base molecules instead of one.
The resulting network will not dissolve in any solvents, and resists all but the strongest chemical reagents. The plurality of OH groups provides hydrogen bonding, useful for adhesion to polar surfaces like glass, wood, etc.
–Robello (accessed 2011): Epoxy Polymers
The … organic materials in [some] meteorites probably originally formed in the interstellar medium and/or the solar protoplanetary disk, but was subsequently modified in the meteorites’ asteroidal parent bodies. … At least some molecules of prebiotic importance formed during the alteration.
Herd et al., 2011: Origin and Evolution of Prebiotic Organic Matter As Inferred from the Tagish Lake Meteorite
Amino acids are the building blocks of life as we know it. They can be formed from abiotic (non-biological) chemical reactions (in a jar with electricity for example). It’s been known for a while that amino acids can be found on comets and asteroids, but now this fascinating article suggests that a lot of the chemical reactions that created these precursors to life happened on the asteroids themselves. Then when the asteroids bombarded the Earth, the seeds of life were delivered. More details here.

This, of course, is just one of several hypotheses about the origin of life on Earth: livescience.com outlines seven.