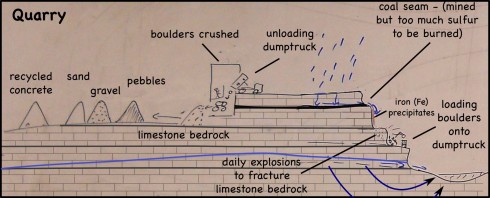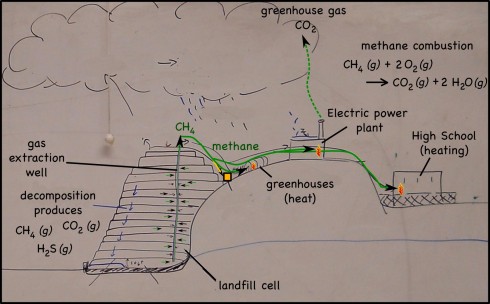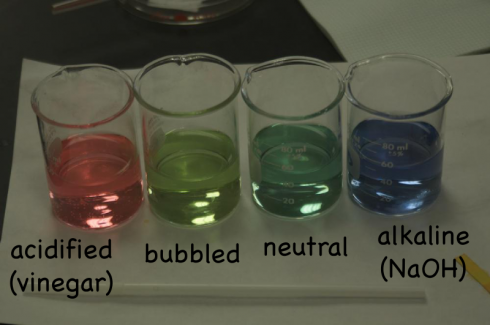
CO2 + H2O —-> H2CO3
This useful little reaction, where carbon dioxide reacts with water to produce carbonic acid, came up in my middle school class when we talked about respiration, it’ll come up soon in environmental science with the effects of carbon dioxide on the oceans (acidification), and it offers the opportunity to discuss pH and balancing chemical reactions in chemistry.
The middle school class did the neat little experiment where students blow bubbles in water (through a straw), and the carbon dioxide in their breath reacts with the water to slightly acidify it. A little universal pH indicator in the water (or even cabbage juice indicator) shows the acidification pretty well if you make sure to keep a standard nearby so students can see the change in color.
The fact that the CO2 in your breath is enough to acidify water begs the question — which was asked — how much of the air you exhale is carbon dioxide? According to the Oak Ridge Carbon Dioxide Information Analysis Center’s FAQ page, it’s concentration is about 3.7% by volume. Which is a lot more than the 0.04% average of the atmosphere.
Of course if you really want to talk about the pH you need to get into the acid equilibrium and the dissociation of the carbonic acid to produce H+ ions; you can get the these details here.