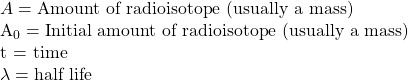Since we most commonly talk about radioactive decay in terms of half lives, we can write the equation for the amount of a radioisotope (A) as a function of time (t) as:
where:
To reverse this equation, to find the age of a sample (time) we would have to solve for t:
Take the log of each side (use base 2 because of the half life):Use the rules of logarithms to simplify:
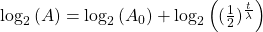
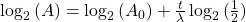
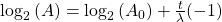
Now rearrange and solve for t:
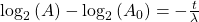
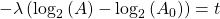
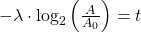
So we end up with the equation for time (t):
Now, because this last equation is a linear equation, if we’re careful, we can use it to determine the half life of a radioisotope. As an assignment, find the half life for the decay of the radioisotope given below.
| t (s) | A (g) |
| 0 | 100 |
| 100 | 56.65706876 |
| 200 | 32.10023441 |
| 300 | 18.18705188 |
| 400 | 10.30425049 |
| 500 | 5.838086287 |
| 600 | 3.307688562 |