I needed a little icon of flames to show the methane from the landfill being burned for heat. So I googled, “svg flames” and ran into the free svg blog. Their svg images are aimed at scrapbookers, but they’ve got some good ones, and they’re free.
Middle and High School … from a Montessori Point of View

I needed a little icon of flames to show the methane from the landfill being burned for heat. So I googled, “svg flames” and ran into the free svg blog. Their svg images are aimed at scrapbookers, but they’ve got some good ones, and they’re free.

NASA thinks their rover has found veins of gypsum on Mars. If they have, it will be an excellent indication that there was once standing water on Mars — gypsum is usually precipitated in evaporating lakes — and will excite the search for life on Mars.

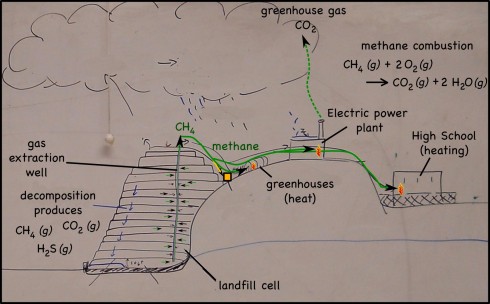
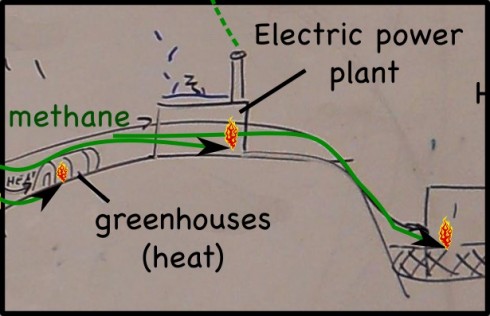
Decomposing waste in landfills produces quite a lot of methane gas (CH4). Perhaps better known as natural gas, methane is one of the simplest hydrocarbons, and a serious atmospheric pollutant (it’s a powerful greenhouse gas). In the past the methane produced was either released into the atmosphere or just burned off.

I remember seeing the offshore oil rigs burning natural gas all night long — multiple miniature sunrises on the horizon — in the days before the oil companies realized they could capture the gas and sell it or burn it to produce energy. The landfill companies have realized the same thing. So now, wells pockmark modern landfills and the methane is captured and used.

First, of course, the hydrogen sulfide gas (H2S), is separated from the methane — H2S produces acid rain, so it’s emissions are limited by the EPA — then, the gas from the landfill we visited, is piped to:

You may have noticed the common theme of all these uses of natural gas: it has to be burned to be useful. The combustion reaction is:
CH4 (g) + 2 O2 (g) —-> CO2 (g) + 2 H2O (g)
which produces carbon dioxide (CO2) that is also a greenhouse gas, but is, at least, not nearly as powerful at greenhouse warming as is methane.

The cliffs of the quarry were stained red. Blood, seeping out from between the bedding planes between layers of rocks, might have left similar traces down the sides of the near-vertical cliffs’ faces. But these stains are actually made of iron.
Rain falling on the land above the quarry, seeps into the ground. There it moves downward through the soil, leaching out some of the minerals there, but going ever downward. Downward until it meets a layer of soil or rock that it can’t get through. Clay layers are pretty impermeable, though in this case it’s a layer of coal. The water can’t move through the near-horizontal coal seam very fast, so instead it moves sideways across, and eventually seeps out onto the cliff face.
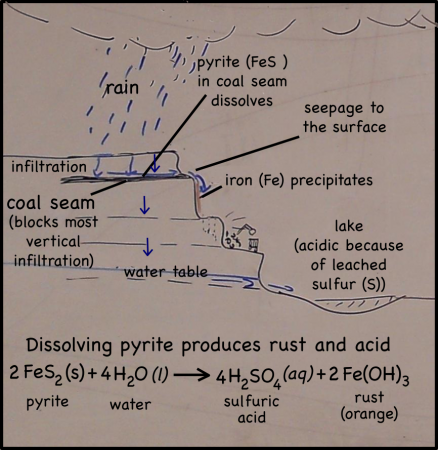
The seeping water still has those minerals it dissolved in the soil. It also has more dissolved minerals from the coal it encountered too. Coal forms in swamps when trees and other plants fall into the waters and are buried before they can completely decompose. Decomposition is slow in stagnant swampy waters because most of the insects and microorganisms that do the decomposing usually need oxygen to help them with their work. Stagnant water does not circulate air very well and what little oxygen gets to the bottom of the swamp-water is used up pretty fast. You could say that conditions at the bottom of the swamp are anoxic (without oxygen), or reducing.
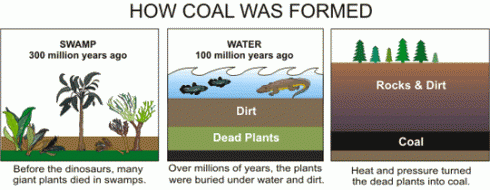
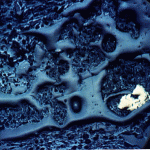
Iron in air will rust as it reacts with water and oxygen — rust is the red mineral hematite (Fe2O3) that you see on the walls of the quarry. Iron in a reducing environment, on the other hand, will form minerals like pyrite (FeS2). According to our guide, the thin coal seam in the quarry has a fair bit of pyrite. In fact, because of the pyrite, the coal has too much sulfur for it to be economical to burn. Like the landfill gas, hydrogen sulfide, burned sulfur turns into sulfur dioxide, which reacts with water droplets in the air to create acid rain so sulfur emissions are regulated.
The water that seeps along and through the coal seam will dissolve some of the pyrite, putting iron into solution. However, the iron will only stay dissolved as long as the water remains anoxic. As soon as the high-iron water is exposed to air, the iron will react with oxygen to create rust. Thus the long stains of rust on the cliff walls show where the water emerges from underground and drips down the cliff face.


We’ve seen the precipitation of iron (rust) as a result of changes in redox (oxidizing vs reducing) conditions before: on the sandbar on Deer Island in the Gulf of Mexico; in the slow streams along the Natchez Trace Park‘s hiking trails in Tennessee. Iron precipitation is an extremely common process in natural environments, and it’s easily noticeable. Just look for the red.

A “symbol” grows in its own way, out of the facts
— Saul Bellow (1963). (via Butler, 2011 in The Paris Review).
Bruce McAllister wrote 150 authors asking if they intentionally put symbolism in their writing. The year was 1963 and McAllister was 16 at the time. Sarah Butler has posted some of the 75 responses McAllister received.
The responses are quite facinating and quite diverse. One common theme, though, was well expressed in the answers to the question, “Do you feel you consciously plan and place symbolism in your writing?”
That would at least be our address if we were on the “extended” Manhattan Street Grid, according to this website.
Just goes to show that everything is relative.

The Voyager 1 spacecraft is rapidly approaching the space between the stars, where the Sun’s solar wind is pushed back by the interstellar magnetic cloud.
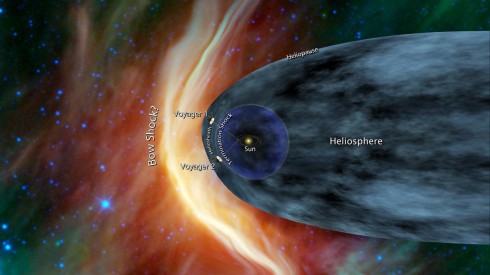
How far will it go? The spacecraft have enough power to last until 2020, when it will be about 20 billion kilometers from the Sun (it’s now about 17.8 billion km away). In 40,000 years it will drift “within 1.6 light years … of AC+79 3888, a star in the constellation of Camelopardalis” that’s about 17.6 light years away.
Consider the 40,000 years it will take Voyager to travel 17.6 light years, and the distance to Keppler 22-b, a recently discovered “Earth-like” planet that’s about 600 light years away.

Keppler 22-b is in the liquid water zone of it’s solar system: far enough away from its sun that water on its surface will not just boil away from the heat, yet close enough that the water does not just freeze solid instead. Liquid water is a key necessity for all life as we know it.
Gum is an effective booster of mental performance, conferring all sorts of benefits without any side effects. … chewing gum is often a better test aid than caffeine. [However] gum chewers only showed an increase in performance during the first 20 minutes of testing.
— Lehrer, 2011: The Cognitive Benefits of Chewing Gum in Wired.
Jonah Lehrer has a fascinating article on, how chewing gum improves mental performance“. It does not seem to matter what type of gum, just as long as you’re chewing.
The benefits (briefly and probably overly simplified) of chewing gum:
On the other hand, while chewing might be good for most types of memory, one study found that chewing, and other rhythmic tasks reduces short-term recall of long lists (Kozlov et al., 2011).
Lehrer cites a 2004 review of the research on gum and memory, which describes chewing gum as, “a functional food with function but no food” (Scholey, 2004).
The takehome message for using gum while taking tests:
When taking a test, save the gum for the hardest part, or for those questions when you feel your focus flagging. The gum will help you concentrate, but the help won’t last long.
— Lehrer, 2011: The Cognitive Benefits of Chewing Gum in Wired.
