
NPR has a nice article on how physicists come up with new elements (in supercolliders) and then name them.
Middle and High School … from a Montessori Point of View
Tiny quantities of dysprosium can make magnets in electric motors lighter by 90 percent, while terbium can help cut the electricity usage of lights by 80 percent.
–Lifton (2010): The Battle Over Rare Earth Metals
There has recently been a bit of a furor over the fact that, currently, China produces 90% of the world’s rare earth metals. Special properties of these elements are making them extremely important in a lot of high-tech and alternative energy technologies.
Fiber-optic cables can transmit signals over long distances because they incorporate periodically spaced lengths of erbium-doped fiber that function as laser amplifiers. Er is used in these laser repeaters, despite its high cost (~$700/kg), because it alone possesses the required optical properties.
–Haxel et al., 2005: Rare Earth Elements—Critical Resources for High Technology
The rare earths are so chemically similar that they’re lumped together in one corner of the periodic table, which is why they have not been used a lot until now. Only recently has their influence on elecromagnetic systems been discovered. Wikipedia has a good list of the elements with some of their uses.

Many people are worried about one country controlling so much of a single resource, especially since China cut its export quotas earlier this year. Fortunately, rare earth metals are found in places other than China, and, as the demand continues to outstrip supply, it’s just a matter of time for high prices to to bring more mining and recycling projects into production.

We could have been talking about the nuclear meltdowns in Japan, but I’m not sure. Our conversations tend to wander. I remember trying to explain where the carbon atoms, that are so essential for life, came from. It’s been a while since we saw this topic, so I figured it wouldn’t hurt to go it over again. And then I found this wonderful image of the Tycho supernova from the Chandra space telescope. Supernovas are where the heaviest atoms are formed.
In the beginning … the big bang created just the smallest elements, hydrogen and helium. But even these tiny things have gravity, so they pull each other together until there’s so much stuff that the pressure at the center of the clump is enough to fuse hydrogen atoms together.
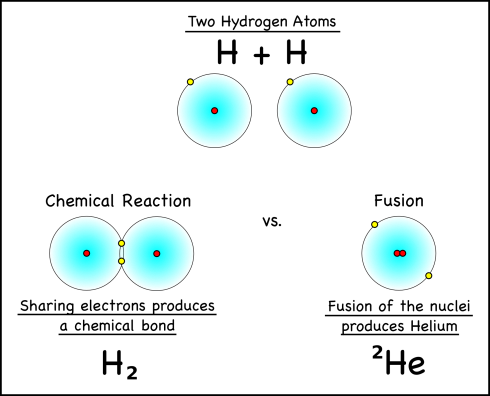
Now fusion is easy to confuse with chemical bonding that occurs around us every day. After all, the hydrogen in the atmosphere is usually in the form of H2, which is two hydrogen atoms bonding together by shared electrons.
With fusion, on the other hand, the single protons that make up the nuclei of the hydrogen atoms are pushed together to create a bigger atom, helium. I say pushed together, because it takes a lot of pressure to fuse atomic nuclei. And it also releases a lot of energy. Notice all that heat and radiation that comes from the Sun? All that energy was created by the fusion of hydrogen atoms; the smallest element, hydrogen, fuels the stars.
The huge amounts of energy released by fusion makes fusion power one of the holy grails of nuclear energy research. If we were able to create and control self-sustaining fusion reactions, just like what happens in the Sun, we would have a source of tremendous energy. There is a lot of research in this area. Some people have figured out how to build fusion reactors in their basements, but these use a lot more energy than they produce so they’re not very useful as a power plant (Barth, 2010). The ITER reactor, currently being built in France, aims to be the first to produce more electricity than it uses.
Now back to the stars. Hydrogen atoms fuse to form helium, but it takes a lot more pressure to create larger atoms: carbon has six protons, nitrogen seven, and oxygen eight. These elements are essential for life (as we know it). The only time stellar forces are great enough to produce these are when stars explode; an exploding star is said to have gone nova. Bigger atoms, like iron (26 protons), gold (79 protons), and uranium (92 protons) need even greater forces, forces that only occur when the largest stars go supernova.
So if these elements are only produced in novae and supernovae, how did they get to Earth? How did they get into your DNA?
Well when stars explode, a lot of these newly formed elements are blasted off into space. It’s a sort of cosmic dust. We could even call it stardust. It’s matter, just like the hydrogen and helium from the big bang, only bigger, which means they have more mass, which means they have more gravity.
The gravity pulls the stardust together with the hydrogen and helium sill floating around in space (there’s a lot of it), to form new stars, and, now that there are the larger elements to create them, rocks, asteroids, and planets.
So, if you think about it, some stars needed to have been formed, lived their lives (which consists of fusing hydrogen atoms until they run out), and exploded to create the matter that makes up the planets in our solar system and the calcium in our bones, the sodium in our blood, and the carbon in our DNA.
1. Lots of information about Tycho’s Star on SolStation.com.
Hydrogen is an alternative source of fuel (alternative to fossil fuels), but it can be produced from renewable or non-renewable sources. PBS and Scientific American Frontiers have a nice video on hydrogen fuel. They first visit a lab producing hydrogen fuel cells. The second part of the program visits Iceland is trying to use geothermal energy to create the hydrogen. They also discuss producing hydrogen from solar-electric and algae.
The BBC has an excellent video demonstration by Maggie Aderin-Pocock of how to demonstrate how additional carbon dioxide in the air results in global warming. She uses baking soda and vinegar to create the CO2 and lamps for light (putting the bottles in the sun would work just as well). You’d also probably want to use regular thermometers in the bottles if you don’t have ones that connect to your computer.
NOVA’s program The Elegant Universe has an excellent website where the entire three hour video is available for free (with a full screen option). They have also broken the video up into segments and have a great teachers’ page which summarizes what’s in each segment.
Created in 2003, when string theory was making it’s big splash in the popular consciousness, The Elegant Universe starts with Newton’s observations of gravity, shows Einstein’s separate explanations of why gravity works and the nature of the sub-atomic world, and finally delves into string theory which tries to reconcile Einstein’s two theories into a unified whole.
We don’t usually get past Newton in middle school, but this PBS program introduces such a wider and weirder view of the universe that it can help strike the imagination. It also presents complex concepts in an intelligible way.
Tom Leher has a number of really entertaining science related songs. Here he does the elements and someone (unknown unfortunately) has made a video to go with it, where the elements all pop up on the periodic table as he sings. Since there is no apparent pattern to order in which he sings the elements, this is more a “strike the imagination” type thing rather than anything else.

The way sub-atomic particles behave is weird. They don’t fit very well into our everyday experience of the world, but the math and the experimental observations hold up. Chad Orzel has an interesting post on the seven things everyone should know about quantum physics that’s written in language a lay person can understand. This does not make the concepts much easier to grasp intuitively, because, as I mentioned before, quantum mechanics is weird, but it does explain things so we can begin to grasp the big picture of how the universe works. It also helps explain why they’re building the Large Haldron Collider. So though you may not know the answers, you’ll at least have an idea about what it’s about when your students ask.