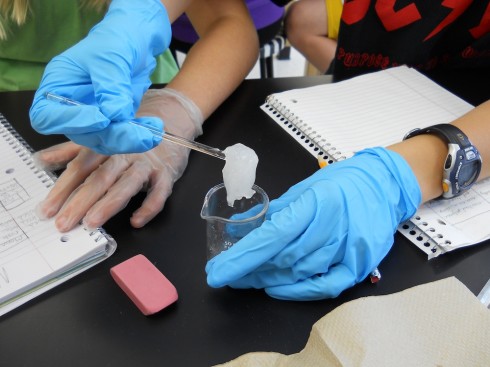
The cliffs of the quarry were stained red. Blood, seeping out from between the bedding planes between layers of rocks, might have left similar traces down the sides of the near-vertical cliffs’ faces. But these stains are actually made of iron.
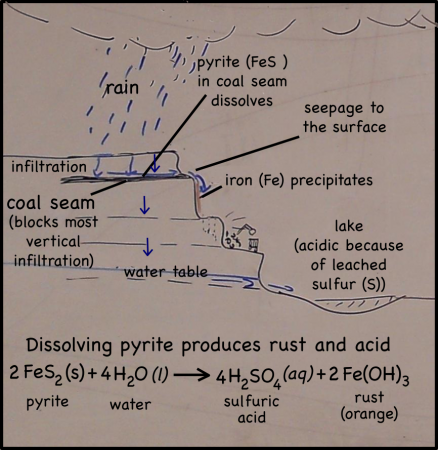
Rain falling on the land above the quarry, seeps into the ground. There it moves downward through the soil, leaching out some of the minerals there, but going ever downward. Downward until it meets a layer of soil or rock that it can’t get through. Clay layers are pretty impermeable, though in this case it’s a layer of coal. The water can’t move through the near-horizontal coal seam very fast, so instead it moves sideways across, and eventually seeps out onto the cliff face.
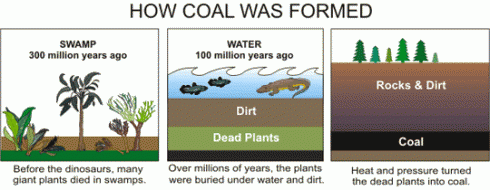
The seeping water still has those minerals it dissolved in the soil. It also has more dissolved minerals from the coal it encountered too. Coal forms in swamps when trees and other plants fall into the waters and are buried before they can completely decompose. Decomposition is slow in stagnant swampy waters because most of the insects and microorganisms that do the decomposing usually need oxygen to help them with their work. Stagnant water does not circulate air very well and what little oxygen gets to the bottom of the swamp-water is used up pretty fast. You could say that conditions at the bottom of the swamp are anoxic (without oxygen), or reducing.
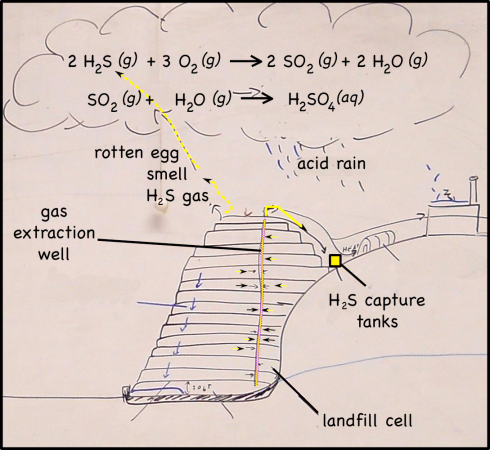
Iron in air will rust as it reacts with water and oxygen — rust is the red mineral hematite (Fe2O3) that you see on the walls of the quarry. Iron in a reducing environment, on the other hand, will form minerals like pyrite (FeS2). According to our guide, the thin coal seam in the quarry has a fair bit of pyrite. In fact, because of the pyrite, the coal has too much sulfur for it to be economical to burn. Like the landfill gas, hydrogen sulfide, burned sulfur turns into sulfur dioxide, which reacts with water droplets in the air to create acid rain so sulfur emissions are regulated.
The water that seeps along and through the coal seam will dissolve some of the pyrite, putting iron into solution. However, the iron will only stay dissolved as long as the water remains anoxic. As soon as the high-iron water is exposed to air, the iron will react with oxygen to create rust. Thus the long stains of rust on the cliff walls show where the water emerges from underground and drips down the cliff face.

Iron precipitate in other environments

We’ve seen the precipitation of iron (rust) as a result of changes in redox (oxidizing vs reducing) conditions before: on the sandbar on Deer Island in the Gulf of Mexico; in the slow streams along the Natchez Trace Park‘s hiking trails in Tennessee. Iron precipitation is an extremely common process in natural environments, and it’s easily noticeable. Just look for the red.