An interesting demonstration of how gold can be extracted from printed circuit boards (PCB’s) and RAM trimmings (which I did not know was a thing). The yield is low, but NileRed gives full detail, including the chemical equations, which is why I may show this to my chemistry class.
Category: Chemistry
Quick Chemical Formulas
While teaching chemistry this year, I’ve needed a quick way to write chemical formulas. The fastest thing to do is just write it out flat–sans sub and superscripts–like Na+, or CO2-. But that’s not pretty, and introduces another potential element that could confuse.
I found that you can use Lingojam’s sub and superscript generators to copy and paste the official UTF-8 characters for pretty much all the sub and superscripts I need for chemistry, but that takes a while to do.
So instead, I put together, just in time to write my finals, a little ChemEqn app that uses keyboard shortcuts to quickly substitute in the sub and superscripts as you type.
- [Ctrl][number]: give you the number subscripted:
- e.g. [Ctrl][2]: gives ₂
- [Ctrl][Shift][number]: gives superscripts:
- e.g. Ctrl][Shift][2]: gives ²
- [Ctrl][Shift][+]: gives a positive charge (⁺)
- [Ctrl][Shift][-]: gives a negative charge (⁻)
- [Ctrl][>]: gives a forward arrow (→)
- [Ctrl][<]: gives a backward arrow (←)
- [Ctrl][/]: gives the double arrow (⇌)
The method is not perfect, since you still have to decide which comes first when you have both a sub and superscript after an element (O₃²⁻ for example). Another issue is that when I do [Ctrl][Shift][+] and [Ctrl][Shift][-] it zooms into and out of the browser window since we’re using the default zoom shortcuts, but that’s, at least for me, a minor inconvenience.
I do like the app since it still makes for quite readable formulas that can be easily copied and pasted almost anywhere without messing up since it only uses UTF-8 characters that are pretty standard across the web (and most computer programs).
Playing with Electron Configurations
I upgraded the table part of the Electron Configuration Interactive I used in the app I made for Practicing Writing out Electron Configurations. It’s now more interactive and embeddable.
Click on the green cell (in the 3d subshell) to start adding electrons. Clicking on the previous cell will remove electrons.
The full documentation is here.
Electron Configuration Practice
A quick electron configuration practice webpage that lets you enter the symbol for an element and see if you can write out the electron configuration in both the full and noble gas forms.
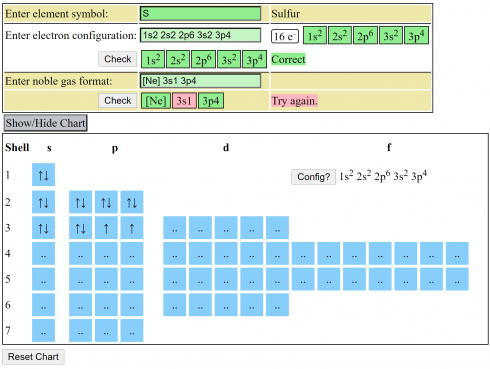
The table at the bottom is a guide to filling the electron shells and orbitals. You can click any of the blue squares to change the number of electrons in the orbital.
Update
An improved version of the lower, table part is here.
History of the Periodic Table
The first video is a quick overview of why Mendeleev’s table of elements was found to be so useful.
The second video goes into more depth about the periods and the groups.
Linearizing an Exponential Function: Radioactive Decay
Using this data for the decay of a radioisotope, find its half life.
| t (s) | A (g) |
| 0 | 100 |
| 100 | 56.65706876 |
| 200 | 32.10023441 |
| 300 | 18.18705188 |
| 400 | 10.30425049 |
| 500 | 5.838086287 |
| 600 | 3.307688562 |
We can start with the equation for decay based on the half life:
where:
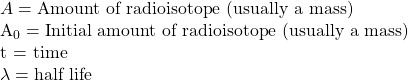
and linearize (make it so it can be plotted as a straight line) by using logarithms.
Take the log of each side (use base 2 because of the half life):Use the rules of logarithms to simplify:
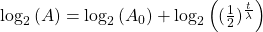
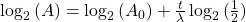
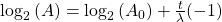
Finally rearrange a little:
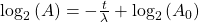
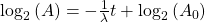
Now, since the two variables in the last equation are A and t we can see the analogy between this equation and the equation of a straight line:
and,
where:
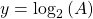
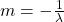
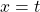
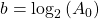
So if we draw a graph with log₂(A) on the y-axis, and time (t) on the x axis, the slope of the line should be:
Which we can use to find the half life (λ).
Radioactive Half Lives
Since we most commonly talk about radioactive decay in terms of half lives, we can write the equation for the amount of a radioisotope (A) as a function of time (t) as:
where:
To reverse this equation, to find the age of a sample (time) we would have to solve for t:
Take the log of each side (use base 2 because of the half life):Use the rules of logarithms to simplify:
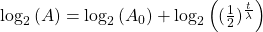
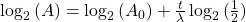
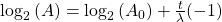
Now rearrange and solve for t:
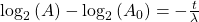
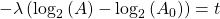
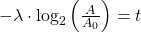
So we end up with the equation for time (t):
Now, because this last equation is a linear equation, if we’re careful, we can use it to determine the half life of a radioisotope. As an assignment, find the half life for the decay of the radioisotope given below.
| t (s) | A (g) |
| 0 | 100 |
| 100 | 56.65706876 |
| 200 | 32.10023441 |
| 300 | 18.18705188 |
| 400 | 10.30425049 |
| 500 | 5.838086287 |
| 600 | 3.307688562 |
Atom Board: Montessori Work
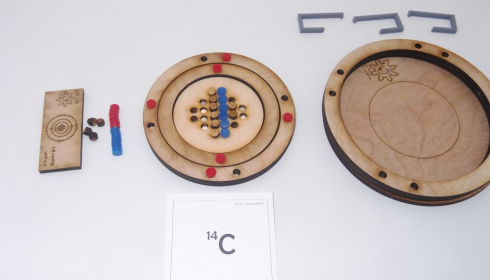
These atom boards worked very well for practicing how to interpret atomic symbols. The protons (blue) and electrons (red) are magnetic so they snap into place quite satisfyingly. Their poles are oriented so that the electrons will only attach properly to the slots in the electron shells and the protons only attach the right way up to the nucleus. The neutrons are wooden and non-magnetic.
Procedure for Building an Atom
Nucleus
Step 1: Number of protons (+ charge).
- The number of protons is given by the element name. Carbon will always have six protons, Hydrogen will have one proton. I have students memorize the first twenty elements in the correct order, so they can quickly determine the atomic (proton) number.
- 14C: Protons = 6+
Step 2: Number of neutrons.
- Neutrons = atomic mass – number of protons
- The atomic mass is given at the top left corner of the atomic symbol: 14 in the example above for 14C.
- 14C: Neutrons = 14 – 6 = 8
Electron Shells
Step 3: Number of electrons (- charge).
- Electrons = number of protons – charge
- The charge is given to the top right of the atomic symbol. In this case, there is no charge
- 14C: Electrons = 6 + 0 = 6
Step 4: Electron Shells
- Electrons go in shells around the nucleus.
- Start with the smallest shell, fill it, and then add the next shell until you’ve placed all of the electrons.
- The first shell can hold only 2 electrons, the second shell can hold 8, and the third 8. The electron configuration tells how many electrons are in each shell.
- 14C: Electron configuration: 2-4

They’ve also turned out to be useful when explaining ionic bonding. Since it’s easy to add or remove electron shells, you can clearly show how many electrons can be donated or received to figure out how many atoms are involved in the reactions.